The Energy Principle
The Energy Principle
The basis of the energy principle can be described with the statement, "energy can neither be created nor destroyed." Thus, energy may only flow from one system to its surroundings. The observable universe is comprised of this system and everything else not in the system called the surroundings. The energy principle is used to describe changes in energy on a system. These energies can take numerous different forms including Kinetic Energy, Potential Energy, Chemical Energy, Rest Energy, and Thermal Energy. Creating boundaries allows for the conservation of these different types energy in that energy is not lost nor is it gained, it is simply transferred into other forms. Any energy moving over the boundaries therefore can be accounted for having been transferred from a system to its surroundings and vice versa. This notion is the core idea informing the Energy Principle: The change in energy of the system should be equal to the energy inputs from surroundings.
The Main Idea
The Energy Principle, also referred to as The First Law of Thermodynamics, defines the transfer of energy between systems. It is defined with the fact that the change of the energy of system is equal to the work surrounding in addition to heat transfers from the surroundings as well. This principle can be modeled by the equations:
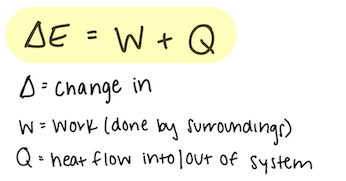
.

How will we use the energy principal?
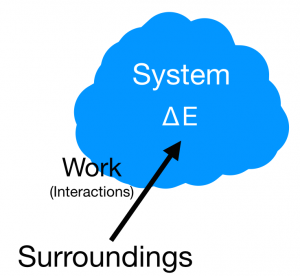
The first step in using the Energy Principle is to identify which initial and final energies have values in the energy equation. You can eliminate the values that are not present in the equation for the specific problem in order to simplify the solution. This form of the energy principle is used to update/predict the future energy of a particular system based on the interactions the system has with its surroundings.
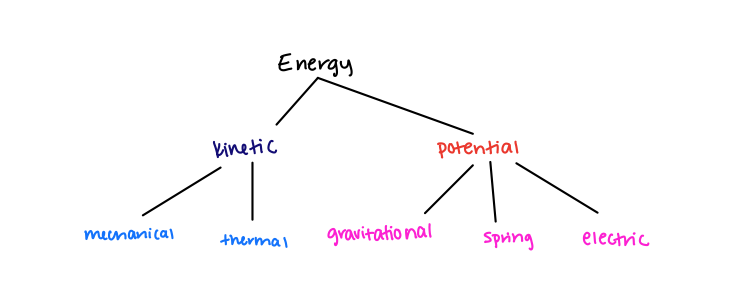
The total energy may have several components to it. Mainly in this course, you will be dealing with kinetic energy and potential energy. Each of these categories can be further defined into mechanical kinetic energy, gravitational potential energy, spring potential energy, etc. Its up to you to determine which type of energy corresponds to the problem you're working on!
.
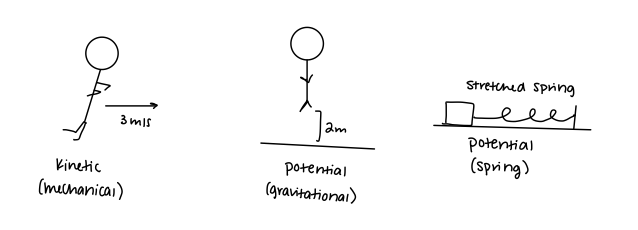
Single Particle vs Multi Particle Systems
The basic equation involving only kinetic and potential energy will often look like the following equation when modeling a single particle system and its surroundings:
KEfinal + U(potential energy)final = Work(surroundings) + Q(heat) + KEintial + U(potential energy)initial
For a multi-particle system: Esystem=(K1+K2+K3+…)+(U1,2+U1,3+U2,3+…)
The equations involving more energies can be seen in the Mathematical Models section.
Click here for a demonstration of the Energy Principle
Mathematical Models
NOTE: These are a lot of equations, but don't get overwhelmed. You simply have to pick and choose which ones are necessary for the problem given; examples are shown below. For in-depth explanations on each type of energy, look at the associated pages on the Main Page or click on the links in the 'See also' section below.
The Energy Principle
EQ 1: [math]\displaystyle{ {∆E} = {Q + W} }[/math] where [math]\displaystyle{ {Q} }[/math] is heat and [math]\displaystyle{ {W} }[/math] is the amount of work acting on the system.
EQ 2: [math]\displaystyle{ {∆E} = {∆K + ∆E_{Rest} + ∆U + ∆E_{Thermal}} }[/math] - the different types of energy that can be associated with a given particle in a system. Not all have to be present. These terms will vary based on the internal properties of the system being observed.
EQ 3: [math]\displaystyle{ E_{Rest}=mc^2 }[/math] - Rest Energy, where m is the mass and c is the speed of light. This type of energy describes the inherent energy contained within an object arising from chemical makeup. Rest energy will only ever change if the system being observed is at an atomic level where particles tends to change identities spontaneously during interactions with surroundings.
EQ 4: [math]\displaystyle{ K=\frac{1}{2}mv² }[/math] - Kinetic Energy, where m is the mass and v is the velocity (for speeds less than the speed of light).
EQ 5: [math]\displaystyle{ ∆E_{Thermal} = mC∆T }[/math] - Thermal energy, were m is the mass, C is the specific heat of water (4.2 J/g/K), and T is temperature. This energy describes the change in average energy of all the particles that comprise a system.
Potential Energy Equations:
EQ 6: 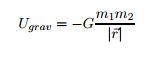 . - for use when not near the Earth's surface. Where m1 = mass of object 1, m2 = mass of object 2, and r = the linear distance separating the two.
. - for use when not near the Earth's surface. Where m1 = mass of object 1, m2 = mass of object 2, and r = the linear distance separating the two.
EQ 7: ![]() . -because the object is near Earth's surface acceleration will be 9.8m/s^2. Where m is the mass of the system.
. -because the object is near Earth's surface acceleration will be 9.8m/s^2. Where m is the mass of the system.
EQ 8: 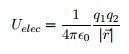 . - for use for electric potential energy. Where q1 = the charge of particle 1, q2 = the charge of particle 2, and r = the distance separating the two interacting fields.
. - for use for electric potential energy. Where q1 = the charge of particle 1, q2 = the charge of particle 2, and r = the distance separating the two interacting fields.
EQ 9: 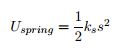 . - for use in spring potential energy. Where ks is the spring stiffness constant, s is the distance the spring is stretched from equilibrium.
. - for use in spring potential energy. Where ks is the spring stiffness constant, s is the distance the spring is stretched from equilibrium.
A Computational Model
This is a good visualization of kinetic, potential, and total energy.
Energy of a single particle
Types of point particles:
- 1. Electrons
- 2. Protons
- 3. Neutrons
Masses of point particles:
- 1. Electron mass = 9.109 e -31 kg
- 2. Proton mass = 1.6726 e -27 kg
- 3. Neutron mass = 1.6750 e -27 kg
[math]\displaystyle{ E_{rest}=mc^2 }[/math]
- Note that rest energy is not dependent on particle speed
The energy of a particle with mass m:
[math]\displaystyle{ E_{particle}=γmc^2 }[/math]
The kinetic energy K of a particle:
[math]\displaystyle{ K=γmc^2-mc^2 }[/math]
- Note that kinetic energy in this form has to be used when the particle is moving near the speed of light
- If v<<c, then [math]\displaystyle{ K=\frac{1}{2}mv² }[/math]
Change in rest energy can be useful when finding the energy change of a decay and of fission
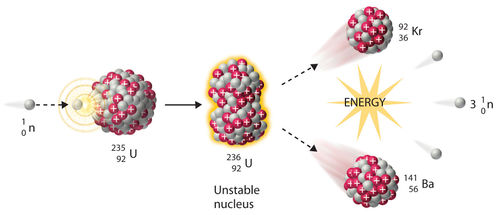
- Uranium nucleus that undergoes fission splits into a bunch of neutrons and a few daughter nuclides.If you trap them all and weigh them the total mass is slightly less than the Uranium nucleus you started with. The missing mass has been converted to energy
Example:
A proton moves at 0.950c. Calculate its (a) rest energy, (b) total energy, and (c) kinetic energy.
- (a) [math]\displaystyle{ E_{rest}=mc^2 }[/math] = (1.67e-27 kg)(3e8 m/s)^2 = 1.5e-10 J
- (b) [math]\displaystyle{ E_{particle}=γmc^2 }[/math] = (1.5e-10 J)/((1-(0.950c/c)^2)^(1/2)) = 4.81e-10 J
- (c) [math]\displaystyle{ K=γmc^2-mc^2 }[/math] = 4.81e-10 J - 1.50e-10 J = 3.31e-10 J
Examples
Simple
A ball weighing 12.6 kg falls off of a cliff that is 98.2 m high. What is the final velocity of the ball when it reaches the ground?
Solution:
In this scenario, the work done from the surroundings is equal to zero and the transfer of heat is zero as well. Therefore, ΔEsys= 0. As shown in the solution below the initial kinetic energy is equal to zero because there is no initial velocity and the final potential energy is equal to zero because the final height is zero relative to the reference point at the base of the ground. The only potential energy needed for the solution of this problem is gravitational potential energy near the surface of the Earth.
ΔKE + ΔU = 0
Thus,
KEf + Uf = KEi + Ui
As described above,
KEi=0 , Uf=0, Velocityi=0 and the unknown: Velocityf=?
1/2*m*(Velocityf)^2 + 0 = 0 + m*g*heighti; The masses algebraically cancel out
Substituting in the values in the problem,
1/2*(Velocityf)^2 = g*heighti
Finally solving for Velocityf will give you,
Velocityf = 43.87 m/s
Difficult
A 90 kg rock is moving with a speed of 4.7 m/s. A machine tries to slow it down by pushing it in the opposite direction of its movement for 2.7 m at an average force of 215 N. What is the speed of the rock after the machine stops pushing the rock?
Solution:
There is no transfer of heat so Q is eliminated from The Energy Principle equation. The equation you would use is ΔEsys= W. Additionally, the work is negative in this scenario because the force is in the opposite direction of momentum. There is no potential energy of any sort. Work = Force(exerted by surroundings/machine) * distance.
Thus,
ΔE = Work
ΔU = 0
ΔE = W = ΔKE + ΔU
Work = KEf - KEi
Work = Force * distance = (215 N) * (2.7 m) = 580.5 N*m
Velocityi = 0, Velocityf = ?
1/2*m*(Velocityf)^2 = -580.5 + 1/2*m*(Velocityi)^2
Masses cancel out again
1/2*(90 kg)*(Velocityf)^2 = -580.5 + 1/2*(90kg)(4.7 m/s)^2
Therefore after solving for Velocityf,
Velocityf = 3.0315 m/s
Connectedness
The Energy Principle can be used for a variety of situations; the fact that it can tell us something about the work acting on a system with only knowing about what energies are present (and vice versa) is what makes this such a fundamental principle. The energy principle is also used to describe the conservation of energy because energy doesn't just disappear, it's converted into a different form. The initial and final total energies of a system should be the same because energy is never lost, just converted into a different form. These sorts of energy balances are used not only in physics, but are integrated into many areas of engineering where the manufacturing of products is supplied by different types of energy such as thermal, electric, gravitational, etc.
Example: Energy Balances in Chemical Engineering
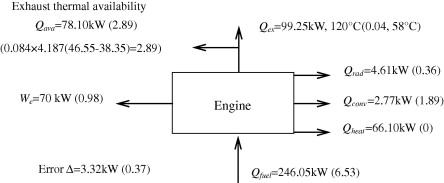
History
The concept of energy and its connection to the amount of work performed goes all the way back to the age of steam engines; physicists and engineers came up with this notion to determine the mechanical and thermal efficiency of their machines. In the 1850's, people like William Thomson and William Rankine began to come up with terms like 'kinetic energy' and 'potential energy' to model the different types of observed forces. After the 1920's, this study of science became to be known as thermodynamics, the science of energy transformations. This led to the laws of thermodynamics, one of which relates to the conservation of energy. William Rankine was the first to discuss the law of the conservation of energy in relation to a more general "energy principle". His discussions and work in this field defined the relationships between energy that we now consider the Energy Principle.
See also
Potential Energy
Rest Mass Energy
Kinetic Energy
Work
Thermal Energy
Gravitational Potential Energy
Conservation of Energy
Spring Potential Energy
Further reading
Matter and Interactions By Ruth W. Chabay, Bruce A. Sherwood - Chapter 6
External links
http://hyperphysics.phy-astr.gsu.edu/hbase/conser.html#coneng
http://hyperphysics.phy-astr.gsu.edu/hbase/enecon.html
https://www.youtube.com/watch?v=-tNQKn0EfBo
https://www.youtube.com/watch?v=30o4omX5qfo
https://www.youtube.com/watch?v=LNk2mUbnKus
https://www.youtube.com/watch?v=5Vfl1uX6kxM
References
http://p3server.pa.msu.edu/coursewiki/doku.php?id=183_notes:define_energy
http://www.texample.net/tikz/examples/earth-orbit/
https://en.wikipedia.org/wiki/Conservation_of_energy
https://en.wikipedia.org/wiki/History_of_energy
Chabay, Ruth W., and Bruce A. Sherwood. Matter and interactions. Hoboken: Wiley, 2015. Print.