Insulators: Difference between revisions
(→Uses) |
|||
| Line 56: | Line 56: | ||
==Uses== | ==Uses== | ||
Insulators are | Insulators are used in many different capacities but always for the same two functions: to limit the flow of electric current or heat. A prime example of insulator usage is the plastics and other polymers used to coat wires. Wires touching could cause short-circuiting and fires, so a thin covering of an insulating material is nearly always placed around them to help prevent these hazards. This also helps protect wires from other elements; for example, power lines are covered for safety but also for protection from outdoor elements, such as rain, which would act as a conductor and would deteriorate the metal wire. | ||
Insulators are also used the in the space between plates in capacitors. The insulator can take the form of air or a thin piece of plastic or other insulating material. The insulator chosen plays a role in the capacitance of the capacitor based on the dielectric constant, a property of the insulating material. The insulator between the plates ensures charge cannot flow from one plate to the other, which allows the crucial ability of capacitors to charge and discharge. | |||
The | |||
==Examples== | ==Examples== | ||
Revision as of 20:58, 18 April 2018
Edited by Raghav Srivastava and Dylan Chiodo on 4/17/16 , Edited by Zinka Bartolek Fall 2016, Edited by Garrett Gresham Spring 2017, Edited by Emma Flynn Spring 2018
Conductors have mobile charged particles that move that can move throughout the material.This is due to a mobile "sea" of charged particles that are not bound to the atoms in the material. Unlike in conductors, the charged particles in insulators are tightly bound to the atoms in the material. Because of this, there can be no charge movement which makes the flow of electric current through the material impossible. Freedom of mobile charges(electrons in metals) all has to due with the valence of electrons in the metal's outer orbitals. A material like copper with one free electron in its outer orbital is a great conductor whereas a material like rubber will not conduct electricity because the polymers it is made of do not have free, mobile electrons. This is why wires are insulated with rubber

The Main Idea
Insulators are a class of materials that restrict the flow of electrons under an applied electric field. When a charge such as an electron is put under an applied field, it will experience a force that causes it to move. However, in an insulator, all electrons are tightly bound to the molecule they belong to, rather than the free-flowing "sea of electrons" in metals. Since there are no free mobile electrons in an insulator, no charges will move through the material. An applied electric field still causes a force on the electrons in the insulator though, and this results in the polarization of the insulator. When an insulator polarizes, the electrons move to the extent that the effective nuclear charge of their proton will allow them to, resulting in induced dipoles. While the effect of each induced dipole is minuscule, the additive induced polarization of the whole material can have a large effect.
How does one determine whether or not a material is going to be an insulator or a conductor? Much of this depends on a material property known as conductivity. Conductivity describes how easy it is for electrons to flow through a material. It is a function of the charge of the mobile charge, the number of mobile charges in a material, and the mobility of the charges. The opposite of conductivity is the property of resistivity which is equal to the inverse of conductivity. This property defines the difficulty of mobile charges to pass through a material. The higher the resistivity of the material, the better an insulator it is.
In the scope of this class, we focus on how insulators are affected by and affect an electric field. When an insulator is placed in an applied electric field, the molecules will polarize even though no charges flow. The induced dipole within the insulator aligns with the electric field with the positive ends facing in the direction of the field. Though the induced dipoles within the insulator affect neighboring molecules with their generated electric field, because the magnitude of the electric field of the dipole is significantly smaller than the magnitude of the applied electric field, we will usually ignore the contribution of each individual dipole in this class. This is able to be neglected by assuming low-density of charge.
Like many things in the real world – there is no such thing as a perfect insulator. A small number of charges will still be able to move, especially when a very high voltage is applied. Insulators are used in electrical equipment to separate conductors and as supports to keep current from flowing where it shouldn’t. In addition, air can be considered an insulator because, in practice, as long as two current carrying wires do not come in contact with one another, electrical current will not pass between them.
A Mathematical Model
Resistivity
Resistivity is the intrinsic property that quantifies how strongly a material opposes the flow of electric current. The units of resistivity are Ohms Meter, and the equation to solve for resistivity is:
[math]\displaystyle{ \rho = R \frac{A}{\ell}, \,\! }[/math] where R is resistance, [math]\displaystyle{ \ell }[/math] is the length of the material and A is the cross sectional area of the specimen.
To calculate resistivity, the size of the material is taken into account along with the resistance. Many objects can claim to have roughly the same resistance, but by taking into the specific dimensions of the material into account we find the resistivity, which is specific to each material. From the equation, it can be concluded that resistivity is an intrinsic property of a material, allowing it to be similar across all specimens of a material. For example, ceramic will have a much high electrical resistivity than copper, however, the resistivity of two ceramic specimens with same dimensions will be the same.
Band Theory
Electrons tend to seek the lowest energy state in their configuration, and the characteristic state, or band, at which the electrons of an element have filled up to is called the Fermi level. Only electrons near the Fermi level are allowed to "jump" to another atom, which is how the electron sea of metals works. Metals have many electrons near their Fermi levels, allowing them to have great conductivity as compared to other materials. The opposite is true for insulators. In an insulator the amount of electrons available for conduction is close to none, meaning a high voltage potential at one end of the insulator will cause little to no change in the electrical structure of the insulator.
Polarization of Insulators
In insulators, all charged particles are tightly bound to the molecules making up the material. When an electric field is applied, the charged particles (electrons) in an insulator shift position slightly but still stay bound to the molecules. The largest distance the charged particles can move is about one atomic diameter, which is 1e-10 m. Because the mobile charge is displaced by such a small distance, the polarization happens very quickly. The whole process can generally take less than a nanosecond to complete.
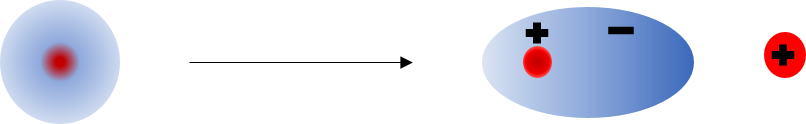
The following image depicts the polarization of a single atom. The red, positively-charged nucleus is surrounded by a region of uniform electron density initially. When a positive point charge is placed to the right of the atom, the electron density shifts to the right as the negatively charged electrons are attracted to the positive point charge. The atom becomes an induced dipole as the right side becomes slightly negatively charged and the left becomes positively charged. Note that the positively charged nucleus is not what moves, but the electron density around the nucleus is what shifts to result in this induced dipole. The same thing happens on the molecular level for molecules in an insulator. In the covalent bonds of molecules, the electrons are shared between the atoms with a region of shared electron density around both nuclei. When a point charge is put near a molecule, the electron density shifts in the same way as it would for a single atom, and a dipole is induced as the electrons shift either toward or away from the point charge.
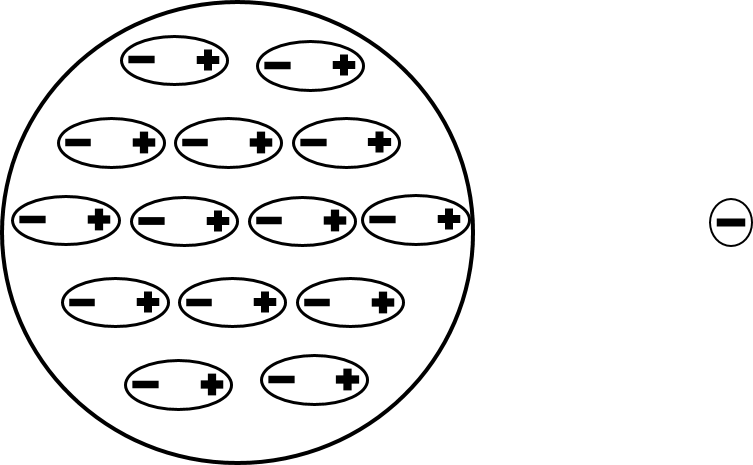
The polarization of an insulator is just the polarization of several moles of atoms or molecules. This image depicts the effect of a negative point charge placed to the right of an insulator. Every atom or molecule in the insulator will be polarized to create several induced dipoles with the positive end facing the negative point charge.
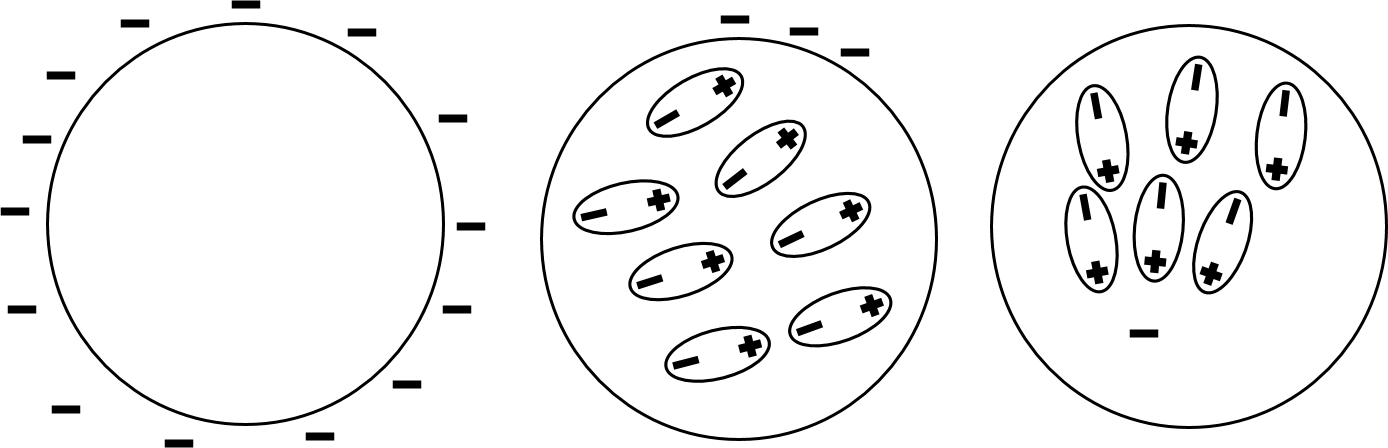
In addition to being polarized by an external charge, insulators can have their own charge. Unlike charges on conductors, this charge is not necessarily evenly distributed throughout the insulator. The image below gives three examples of charge distribution on insulators. The left shows even charge distribution over the outside of the insulator; the middle shows a patch of charge on the outside of the insulator; and the right shows an internal charge in an insulator. Excess charge in patches as well as internal charge can also result in polarization of the insulator's atoms or molecules. However, when the charge is evenly distributed, no dipole is induced as this would cause, in this example, the positive ends to point toward the outside while the negative ends point inward toward each other and repel each other; this is an impossible configuration, so the electron clouds remain evenly distributed. Charges on insulators can polarize other materials; you have most likely felt this when touching a piece of plastic with excess charge on it and feeling your hairs stand on end. This will likely come up in this class in the context of charged insulators polarizing other materials.
Testing Resistivity
To test the resistivity of an insulator we need to look at the breakdown voltage. The breakdown voltage of an insulator is the minimum voltage required to make a portion of the insulator become conductive. This is done by creating a weakened path in the material – a permanent physical or molecular change to the material. Basically the voltage gives the electrons enough energy to be excited. Many will use this to test for the maximum amount of voltage a material can withstand prior to it completely collapsing.
Uses
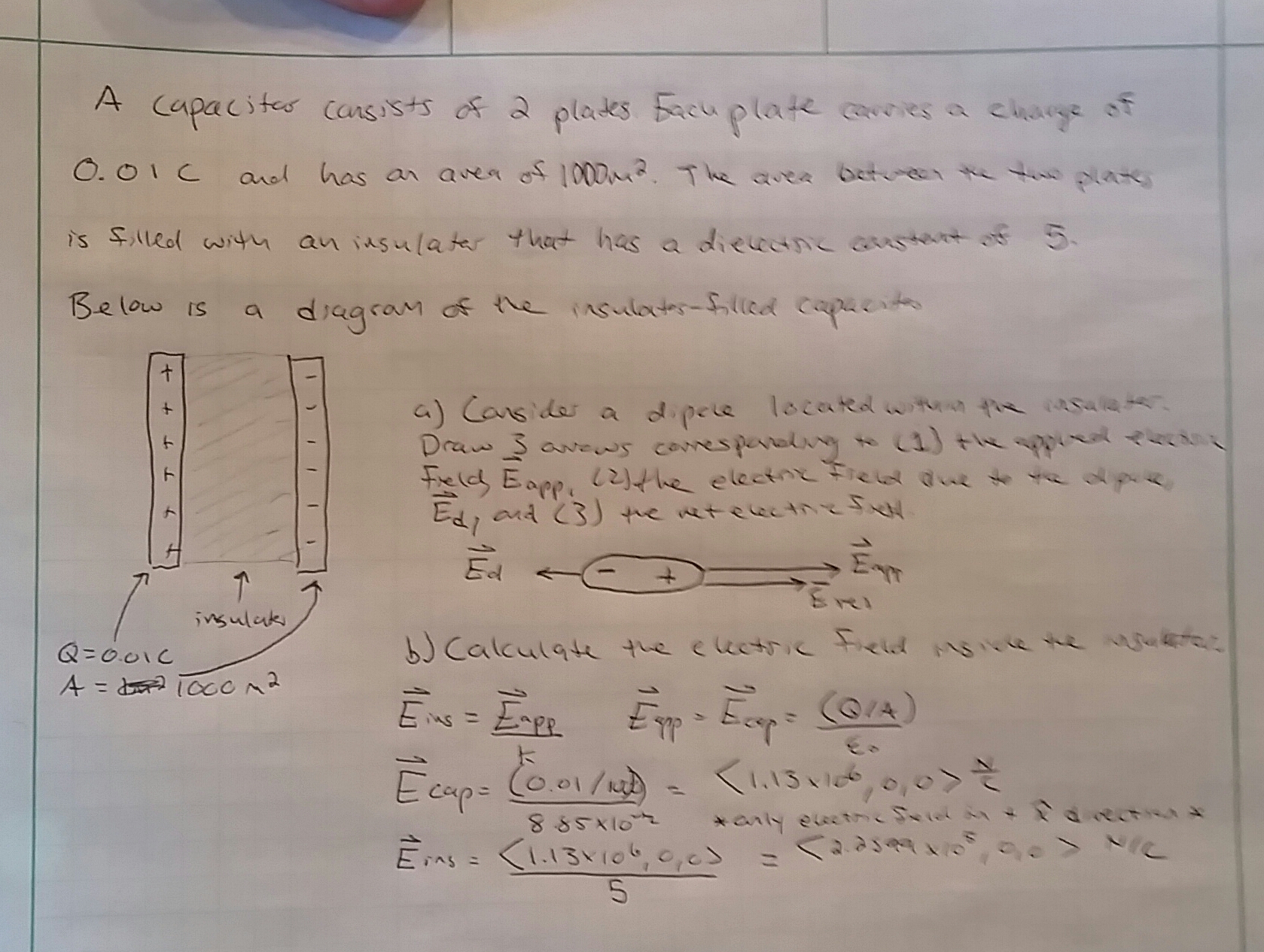
Insulators are used in many different capacities but always for the same two functions: to limit the flow of electric current or heat. A prime example of insulator usage is the plastics and other polymers used to coat wires. Wires touching could cause short-circuiting and fires, so a thin covering of an insulating material is nearly always placed around them to help prevent these hazards. This also helps protect wires from other elements; for example, power lines are covered for safety but also for protection from outdoor elements, such as rain, which would act as a conductor and would deteriorate the metal wire. Insulators are also used the in the space between plates in capacitors. The insulator can take the form of air or a thin piece of plastic or other insulating material. The insulator chosen plays a role in the capacitance of the capacitor based on the dielectric constant, a property of the insulating material. The insulator between the plates ensures charge cannot flow from one plate to the other, which allows the crucial ability of capacitors to charge and discharge.
Examples
Simple
Material A has a conductivity of 200 while material B has a resistivity of 30. Which material is the better insulator?
Since resistivity is the inverse of conductivity, it is simple to see that Material B will be the better insulator.
Middling
You have two capacitors, one filled with insulator A and one filled with insulator B. Insulator A has a dielectric constant of 5 and insulator B has a dielectric constant of 10. Assuming both insulators have the same area and charge on their plates and the same distance between plates, which one would have the greater potential difference across it?
You can read more about potential differences here: Potential Difference. Since both capacitors have plates with the same charge and area, both will have the same applied electric field. The actual electric field inside capacitor A will be greater because it has a lower dielectric constant. Because of this, we will see a greater potential difference by a factor of 2 across capacitor A.
Difficult
Connectedness
Connection to Interests
This topic is very connected to my interests. I really enjoy learning about the physics and chemistry behind material properties. It amazes me to see how chemical properties such as the number of valence electrons in a material can have such a profound effect on a physical property like conductance.
Connection to Major
As a chemical engineer, the topic of insulators can be connected to my major. One of the industries I am considering working in is the materials industry. It is important to understand the chemistry behind a material so that you can know how that material will behave in certain applications.
Power lines
Typically these high voltage wires are insulated by air. Insulator materials are only used when the wires are connecting to a pole or support. Insulators are also required where these lines enter buildings and electrical centers. Ceramics (including glass), are the material of choice in building insulators for power lines, and they typically have an outer coat of gloss to prevent the buildup of condensation.
Antennas
Broadcasting antennas are basically a giant high voltage structure, so they must be insulated from the ground and be protected against lightning. Usually cables are used to break up the voltage and to help prevent short circuiting. Ceramic insulators are commonly used. The insulator is under compression as opposed to the tension that is seen with powerlines.
History
The use of electrical insulators began as soon as the use of electricity began being applied. Thomas Edison was awarded a patent in 1892 for creating an electric conductor. This 'electric conductor' consisted of two materials. The first was a cotton-braid mesh that was placed on the wire to separate it from the insulator. The second was a rubber coating. This combination made it safe to begin working with wires. Since then, the application of insulators has become a major part of our daily lives.
See also
References
Holtzhausen, J.P. "High Voltage Insulators" (PDF). IDC Technologies. Retrieved 2008-10-17.
Grigsby, Leonard L. (2001). The Electric Power Engineering Handbook. USA: CRC Press. ISBN 0-8493-8578-4
Bakshi, M (2007). Electrical Power Transmission and Distribution. Technical Publications. ISBN 978-81-8431-271-3.
http://www.allaboutcircuits.com/textbook/direct-current/chpt-12/insulator-breakdown-voltage/
http://www.engineeringtoolbox.com/resistivity-conductivity-d_418.html