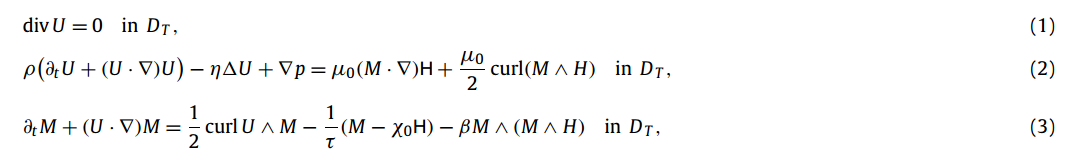Ferrofluids
Claimed by Brandon Chen
Ferrofluids (the combination of ferromagnetic and fluid) are defined as liquid which, when placed within a magnetic field, experience strong magnetizations which can result in incredibly versatile shapes and forms. This phenomenon stems from the dispersal of ferrimagnetic particles (often hematite or other particles containing iron) which help create the composition of the fluid. Typically, these particles are suspended water or another organic solvent.

Significance
Applications

There are a wide array of potential applications for ferrofluids ranging from electronics, to art, to optical engineering, to propulsion. In optics, researchers are attempting to adapt ferrofluids for use in telescopes: the idea is to have a mirror which has capabilities of altering its shape. They are currently used in loudspeakers as a way to cool the voice coil and thus mitigate shifting in the cone. In addition, there are applications in electronic devices; these magnetic fluids can be utilized to seal spinning shafts in hard discs by creating a wall through which minimal debris and other particles can pass. There also exists medical applications through magnetic drug targeting.
The idea behind this it so delivery the drug into the patient's body while its encapsulated by a ferrofluid, then direct the drug into its optimal delivery area using an external magnetic field, and finally release the drug by turning off the field. Lastly, there are potential adaptations in the field of spacecraft propulsion. One of the hallmark effects of ferrofluids are the thin spikes which emerge in the presence of a magnetic field. These spikes, when sharpened to a specific thickness, can emit jets. This effect could be taken advantage of as a way of creating thrust in small objects.
Behavior
The behavior of ferrofluids is dependent on the composition of microscopic particles of magnetic nature, the concentration of these particles, the surfactant, and the carrier fluids. All these can change the viscosity of the fluid, and thus the behavior. In a magnetic field, the ferrofluid "mixture" behaves as one unit and the sharp spikes that can be seen in the display to the right are not actually solid. Furthermore, the fluids display a "change in density" when under the influence of an external magnetic field causing small items, such as pennies, to float when in a strong magnetic field. This behavior translated to real-world applications in the form of magnetic density separation, which can be utilized to separate magnetic from non-magnetic materials.
A Mathematical Model
The following equations can be used to mathematically model the behavior of a ferrofluid when under the influence of an external magnetic field. These equations stem from a combination of the magnetizaton equations (3), magnetostatic equations, and the Navier-Stokes equations (1 and 2).
In these equations, U is the velocity of the fluid, p is pressure, and the other parameters are positive. The magnetic field is H and curl of H = 0.
History
For a long time, researchers were accepting of the fact that solid magnetic materials were either permanent or softly magnetic. However, some dreamed of a stable liquid magnet, and this dream was spurred on by NASA in the 1960s. NASA was confronted with the problem of the lack of gravity on fuel in space which would lead to inefficient burning by the engine. But if the fluid could be controlled by a magnetic field, then the pumping could be regulated and optimized. And in 1965, Steven Papell created the first magnetic fluid (with a kerosene solvent). Yet his creation was never adopted as NASA opted for solid rocket fuel. But research continued with AVCO Corporation and eventually commercial use began in 1968 with the Ferrofluidics Corporation.
Examples of common ferrofluid surfactants
Surfactants are used to cover the magnetic particles within the solvent in order to prevent clumping. The surfactant lowers surface tension between the liquid and solid.
- oleic acid
- tetramethylammounium hydroxide
- citric acid
- soy lecithin
See also
Further reading
- Y. Amirat, K. Hamdache, F. Murat Global weak solutions to the equations of motion for magnetic fluids
- Y. Amirat, K. Hamdache Global weak solutions to a ferrofluid flow model
- Y. Amirat, K. Hamdache, Unique solvability of equations of motion for ferrofluids, Preprint, 2008\
- Y. Cho, H.J. Choe, H. Kim Unique solvability of the initial boundary value problems for compressible viscous fluids
External links
References
- B. Ducomet, E. Feireisl The equations of magnetohydrodynamics: On the interaction between matter and radiation in the evolution of gaseous stars
- G. Lukaszewicz Micropolar Fluids: Theory and Applications
- R. TemamNavier–Stokes Equations (third (revised) ed.)Elsevier Science Publishers B.V., Amsterdam (1984)
- Albrecht, T.; Bührer, C.; Fähnle, M.; Maier, K.; Platzek, D.; Reske, J. (1997). "First observation of ferromagnetism and ferromagnetic domains in a liquid metal". Applied Physics A: Materials Science & Processing 65 (2): 215.