Scattering: Collisions in 2D and 3D: Difference between revisions
No edit summary |
|||
| Line 8: | Line 8: | ||
Unlike normal collisions, atomic and nuclear collisions are far too small to observe the curving trajectories of the interacting particles. The only thing | Unlike normal collisions, atomic and nuclear collisions are far too small to observe the curving trajectories of the interacting particles. The only thing | ||
that can be noticed is the initial and final states of the interaction. Therefore, the method pertained of alpha particles from a radioactive source striking a thin gold foil. | that can be noticed is the initial and final states of the interaction. Therefore, the method pertained of alpha particles from a radioactive source striking a thin gold foil. Since it is only noticed a very small number of atoms are scattered after contact with the gold, it can be determined that the majority of the material is positively charged and when the alpha particle (positive) travels through and approaches close enough to the nucleus, it will repel and then "scatter" into a large angle. | ||
By finding the back-scattering, it shows that atoms are arranged tightly together. Scattering experiments are incorporated in the world of collisions | By finding the back-scattering, it shows that atoms are arranged tightly together. Scattering experiments are incorporated in the world of collisions | ||
| Line 14: | Line 14: | ||
After collision with the gold nucleus, the alpha particle gets deflected some angle θ. The gold nucleus recoils at some angle Φ. It is important to pick optimal time frames ("before" early enough and "after" late enough) so that the two particles are far away enough from each other to avoid their electric potential energies. The speeds in this approach are small in comparison to the speed of light. | After collision with the gold nucleus, the alpha particle gets deflected some angle θ. The gold nucleus recoils at some angle Φ. It is important to pick optimal time frames ("before" early enough and "after" late enough) so that the two particles are far away enough from each other to avoid their electric potential energies. The speeds in this approach are small in comparison to the speed of light. | ||
The case of which Rutherford scattering of alpha particles with gold nuclei is an example of elastic scattering because the initial and final velocity and energy stay the exact same. | |||
==Equations== | ==Equations== | ||
Revision as of 19:07, 9 April 2017
Edwin Zhao - Spring 17
The Main Idea
Scattering (Rutherford Scattering) is a type of experiment that is used to study the structure and behavior of atoms, nuclei, and other small particles. This allows us to understand small particles on a greater level.
Unlike normal collisions, atomic and nuclear collisions are far too small to observe the curving trajectories of the interacting particles. The only thing that can be noticed is the initial and final states of the interaction. Therefore, the method pertained of alpha particles from a radioactive source striking a thin gold foil. Since it is only noticed a very small number of atoms are scattered after contact with the gold, it can be determined that the majority of the material is positively charged and when the alpha particle (positive) travels through and approaches close enough to the nucleus, it will repel and then "scatter" into a large angle.
By finding the back-scattering, it shows that atoms are arranged tightly together. Scattering experiments are incorporated in the world of collisions to be able to study the minute details (structure) of atoms, nuclei, and other tiny particles as the interact with one another.
After collision with the gold nucleus, the alpha particle gets deflected some angle θ. The gold nucleus recoils at some angle Φ. It is important to pick optimal time frames ("before" early enough and "after" late enough) so that the two particles are far away enough from each other to avoid their electric potential energies. The speeds in this approach are small in comparison to the speed of light.
The case of which Rutherford scattering of alpha particles with gold nuclei is an example of elastic scattering because the initial and final velocity and energy stay the exact same.
Equations
[math]\displaystyle{ {p_xi = p_xf} }[/math]
[math]\displaystyle{ {p_1 = p_3 cosθ + p_4 cosΦ} }[/math]
[math]\displaystyle{ {p_yi = p_yf} }[/math]
[math]\displaystyle{ {0 = p_3 cos(90° - θ) + p_4 cos(90° + Φ)} }[/math]
[math]\displaystyle{ {K_f = K_i} }[/math]
[math]\displaystyle{ {\frac{p^2_1}{2m} = \frac{p^2_3}{2m} + \frac{p^2_4}{2M}} }[/math]
Where [math]\displaystyle{ {p_1, p_3, and p_4} }[/math] are all magnitudes of the momenta. It can be remembered that the gold nucleus is initially at rest.
The directions of cosine are used to express vector components to the x-axis.
ex. Final momentum of gold nucleus [math]\displaystyle{ {= \vec{p_4} = |\vec{p_4}| \lt cosΦ, cos(90° + Φ), 0\gt } }[/math]
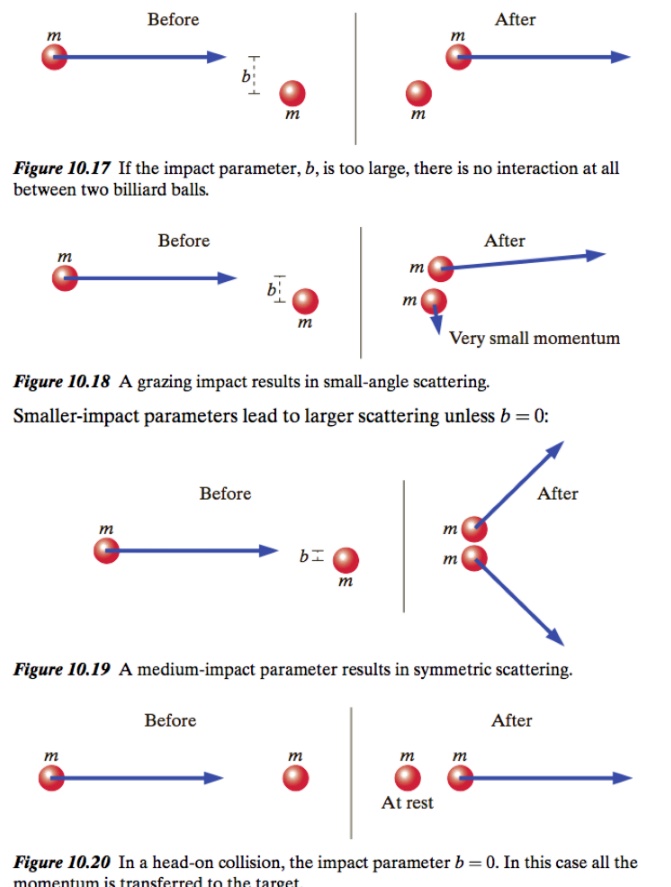
Impact Parameters
Definition: The distance between centers perpendicular to the incoming velocity. Impact parameter is often denoted by the variable b.
A head-on collision has an impact parameter of zero and with equal masses fully transfers the momentum such as with Newton's Cradle. As the impact parameter gets smaller the collision has a larger effect, and an even large deflection angle (scattering).
Elastic collisions between two billiard balls
Examples
Simple
The collision of an alpha particle (helium nucleus) with the nucleus of a gold atom
History
In 1871 Lord Rayleigh published a paper on scattering. Rayleigh scattering is the dispersion of electromagnetic radiation by particles that have a minute radius less than approximately 1/10 the wavelength. It laid the foundation to research on scattering and information we have today.
In Ernest Rutherford's laboratory with Hans Geiger and Ernest Marsden, they experimented with tests of scattering alpha particles with thin metal foils. In 1909, Geiger and Marsden discovered scattering through their gold foil experiments. From this, they discovered that some alpha particles scattered at angles greater than 90°.
See also
[[Category:Collisions] (Main page)
Further reading
Matter and Interactions, Volume I: Modern Mechanics, 4th Edition. (Chapter 10.6)
External links
References
Chabay, Ruth W., Bruce Sherwood. Matter and Interactions, Volume I: Modern Mechanics, 4th Edition. Wiley, 19/2014.