Quantized energy levels
Created by Keller Porter
The nucleus of each every atom creates an electric field, and it is composed of different levels, or stationary orbits, and each one requires a different energy level for an electron to reside there. The electrons in this electric field are in a bound state, requiring energy to be removed from their current energy level. These energy levels are considered quantized. Quantization is a transition from a classical understanding of physical principles to a more modern understanding.
Background
In the 1814, Joseph von Fraunhofer and William Hyde Wollaston discovered that when viewed closely, the spectrum from sunlight contained dark lines. These lines represented wavelengths of sunlight that were not reaching us. These wavelengths were being absorbed by the sun's atmosphere.
If light is shone through a gas, the gas will absorb the specific wavelengths characteristic of the atoms in the gas. If the light were to be put through a prism of light or a diffraction grating, then there would be absorption lines, or places where the wavelength of light had been absorbed into the gas. This process creates something called an absorption spectrum. Similarly, if this same gas was heated to the right temperature, it would emit the same wavelengths that it absorbed before. Putting this emitted light through a prism or diffraction grating would create an emission spectrum. This is the opposite of an absorption spectrum because it shows the emission lines from the gas instead of the absorption lines.
Atom Energy Stability
In 1911, Rutherford came up with his model for the atom. It used all the same components of an atom that we know exist today, but it had one glaring issue. His model lacked stability. Classical electromagnetic theory said that the electrons surrounding the nucleus would quickly collapse because they were emitting electromagnetic waves, causing them to lose energy. If this were true, then the atom as we know it would not be able to exist.
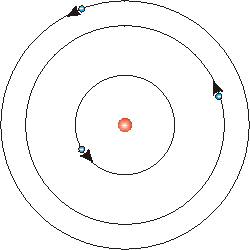
Bohr's model of the atom solved this problem. He proposed that the laws of classical mechanics must be reconsidered. His model said that the electron cloud had stationary orbits, a specific set of orbits for electrons. This differed from the assumption that the electron cloud was just a continuum where the electrons were free to orbit the nucleus. His model was similar to the solar system in that electrons orbit the nucleus like planets orbit the sun. Electrons are held in place by electrostatic forces, and planets are held in place by gravitational forces. The base energy level, called the ground state, is the first stationary orbit. From there, there can be many more levels, denoted [math]\displaystyle{ E_n }[/math], [math]\displaystyle{ n=1,2,3... }[/math]. At the ground state, the energy required to free the electron is greatest. It requires a specific level of energy to excite an electron to another energy level, and energy can be released to bring the electron back down to the ground state.
What are the mathematical equations that allow us to model this topic. For example [math]\displaystyle{ {\frac{d\vec{p}}{dt}}_{system} = \vec{F}_{net} }[/math] where p is the momentum of the system and F is the net force from the surroundings. How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Implications
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
This section contains the the references you used while writing this page