Quantized energy levels
Created by Keller Porter
Revision by Jonathan Ledet Fall 2016
The Main Idea
The electrons around an atomic nucleus are held in orbit by an electric field (created by the positively charged protons interacting with the negatively charges electrons). The atom behaves in such a way that there is only certain spaces, called 'stationary orbits', in which electron orbits can happily exist. This behavior occurs due to the wave-particle duality of electrons and results in a formulaic and regular behavior of energy levels in each stationary orbit. Because the energy levels of each orbit are not a continuous spectrum, and rather exist at discrete values, they are said to be 'quantized'. Quantization is a transition from a classical understanding of physical principles to a more modern understanding.
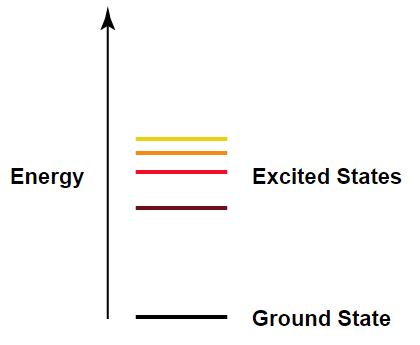
The various levels of energy associated with an atom are described using its principle quantum number (often denoted as [math]\displaystyle{ n }[/math]). A principle quantum number [math]\displaystyle{ n }[/math] of 1 indicates that the electron is in the orbit (or 'shell') closest to the nucleus; this state is of the lowest energy level and is referred to as the 'ground state'. A principle quantum number of greater than 1 indicates an electron in a larger orbit and higher energy level; this state is referred to as 'excited'. Less energy is required to free an electron from an excited state.
If light is shone through a gas, the gas will absorb the specific wavelengths characteristic of the atoms in the gas. If the light were to be put through a prism of light or a diffraction grating, then there would be absorption lines, or places where the wavelength of light had been absorbed into the gas. This process creates something called an absorption spectrum. Similarly, if this same gas was heated to the right temperature, it would emit the same wavelengths that it absorbed before. Putting this emitted light through a prism or diffraction grating would create an emission spectrum. This is the opposite of an absorption spectrum because it shows the emission lines from the gas instead of the absorption lines.
A Mathematical Model
The energy of each level can be found using the formula:
[math]\displaystyle{ E_n = \frac{-2\pi^2me^4Z^2}{n^2h^2} }[/math]
where [math]\displaystyle{ m }[/math] is the mass of an electron, [math]\displaystyle{ e }[/math] is the magnitude of the electric charge, [math]\displaystyle{ n }[/math] is the principle quantum number, [math]\displaystyle{ h }[/math] is Planck's constant, and [math]\displaystyle{ Z }[/math] is the atomic number of the atom. This gives the energy for a specific atom at each energy level. The value will always be negative because electrons in the electron cloud are in a bounded state, so the potential energy is negative.
The radius of a given orbital can be found using the equation [math]\displaystyle{ 2pi = n }[/math]λ[math]\displaystyle{ _n }[/math]
Energy is associated to wavelength by [math]\displaystyle{ E = \frac{hc}{\lambda} }[/math]
While the value for each energy level is set in place, the energy of each individual electron can change. This can happen either by an interaction with another electron or a photon. For an electron, they can jump from the ground state to the fourth level if [math]\displaystyle{ E_{particle} \ge E_4-E_1 }[/math]
A Computational Model
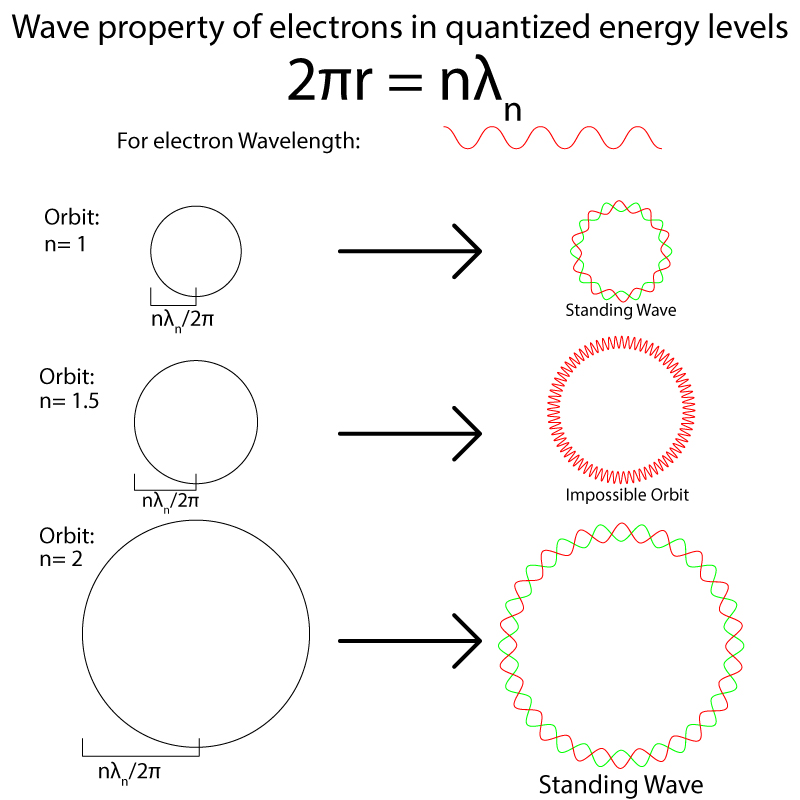
The radii of orbits are restricted to certain values due to the wave nature of electrons. Electrons can only exist happily in standing waves and the waves created by an orbit will only be standing if the wavelength follows the formula [math]\displaystyle{ 2pi*r = n }[/math]λ[math]\displaystyle{ _n }[/math]
Consider a wave of λ = 1 and a series of orbits with varying radii. If that wave is laid along the circular path of the orbit, there are only a few specific circumferences that will allow the wave to fit perfectly, forming a standing wave. Any length where the wavelength does not form a standing wave will cause patterns of interference and a stable orbit would be impossible.
Example: Hydrogen Model
Research done by Johannes Rydberg showed that hydrogen has a ground level energy of [math]\displaystyle{ E_R = 13.6eV }[/math]. With Rydberg's constant, the initial formula for energy levels can be altered to include this. This new formula is:
[math]\displaystyle{ E_n = \frac{-E_R}{n^2} }[/math]
Simple
Will a photon with energy [math]\displaystyle{ E_{photon} = 10.4eV }[/math] be able to bump a hydrogen electron in the ground state to the second level?
Solution: [math]\displaystyle{ E_1 = \frac{-13.6}{1} = -13.6eV }[/math]
[math]\displaystyle{ E_2 = \frac{-13.6}{2^2} = \frac{-13.6}{4} = -3.4eV }[/math]
[math]\displaystyle{ E_2 - E_1 = -3.4eV - -13.6eV = 10.2eV }[/math], so it requires [math]\displaystyle{ 10.2eV }[/math] to bump an electron from the ground state to the second level.
[math]\displaystyle{ 10.4eV \ge 10.2eV }[/math]
Yes, the photon has enough energy to bump the electron from the ground state to the second energy level.
Middling
If an electron orbiting hydrogen starts in the n = 4 orbit and ends in the ground state, how many photons with different energies can the atom emit?
The different possible orbital transitions possible in the atom are (where the numbers indicated are possible values of n):
4 -> 3
4 -> 2
4 -> 1
3 -> 2
3 -> 1
2 -> 1
There are 6 different possible transitions, which correspond to 6 different energy levels the photons emitted from these transitions can have.
Difficult
What is the energy of a Hydrogen electron in an orbit of radius .4761 nm? What form of electromagnetic radiation is necessary to free this electron from its orbit?
1) Using the formula for the radius of the orbit, find the value of n for this electron:
[math]\displaystyle{ r = a_{0}n^2 }[/math] where n =1,2,3... and the Bohr Radius [math]\displaystyle{ a_{0} = 0.0529*10^{-9} }[/math]
[math]\displaystyle{ n^2 = \frac{r}{a_{0}} = \frac{.4761*10^{-9}}{0.0529*10^{-9}} = 9 }[/math]
2) Using the value of n, calculate the energy of the electron:
[math]\displaystyle{ E_{n} = \frac{-13.6 eV}{n^2} = \frac{-13.6 eV}{9} = -1.51 eV }[/math]
3) Set the electron's ionization energy (the energy required to free it from its orbit) equal to the energy of a photon ([math]\displaystyle{ E_{photon} = \frac{hc}{λ} }[/math]) and solve for the wavelength λ:
[math]\displaystyle{ E_{Ionization} = |E_{n}| = 1.51 eV }[/math]
[math]\displaystyle{ E_{Ionization} = \frac{hc}{λ} }[/math]
[math]\displaystyle{ λ = \frac{hc}{E_{Ionization}} }[/math]
[math]\displaystyle{ λ = \frac{(4.14*10^{-15} eV*s)(2.998*10^8 m/s)}{1.51 eV} = .822 μm }[/math]
4) Examine the wavelengths of electromagnetic radiation and determine the form with an interval of wavelengths containing .822 μm:
A wavelength of .822 μm is characteristic of ultraviolet light.
Implications
Until quantization of atomic energy levels occurred, there were a few different experiments that had already been performed that did not make sense. Once researchers discovered the actual way electrons make up the electron cloud as well as the mechanisms that help them jump from level to level, these experiments could be explained. Readings for these three experiments can be found below.
History
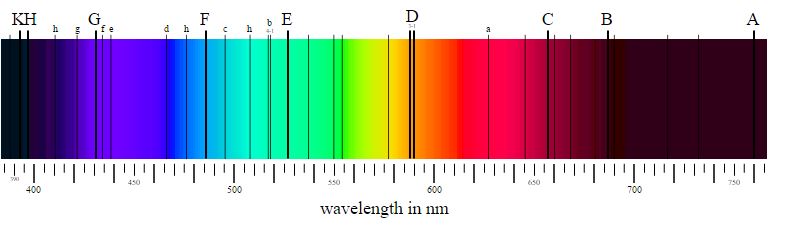
In the 1814, Joseph von Fraunhofer and William Hyde Wollaston discovered that when viewed closely, the spectrum from sunlight contained dark lines. These lines represented wavelengths of sunlight that were not reaching us. These wavelengths were being absorbed by the sun's atmosphere.
In 1911, Rutherford came up with his model for the atom. It used all the same components of an atom that we know exist today, but it had one glaring issue. His model lacked stability. Classical electromagnetic theory said that the electrons surrounding the nucleus would quickly collapse because they were emitting electromagnetic waves, causing them to lose energy. If this were true, then the atom as we know it would not be able to exist.
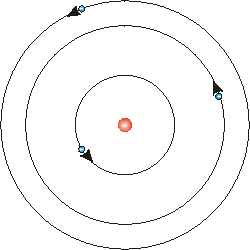
Bohr's model of the atom solved this problem. He proposed that the laws of classical mechanics must be reconsidered. His model said that the electron cloud had stationary orbits, a specific set of orbits for electrons. This differed from the assumption that the electron cloud was just a continuum where the electrons were free to orbit the nucleus. His model was similar to the solar system in that electrons orbit the nucleus like planets orbit the sun. Electrons are held in place by electrostatic forces, and planets are held in place by gravitational forces.
References
- http://hyperphysics.phy-astr.gsu.edu/hbase/bohr.html
- http://www.chemistry.mcmaster.ca/esam/Chapter_3/section_1.html
- http://www.colorado.edu/UCB/AcademicAffairs/ArtsSciences/physics/TZD/PageProofs1/TAYL05-144-167.I.pdf
- "Emission spectrum-H" by Merikanto, Adrignola - File:Emission spectrum-H.png. Licensed under CC0 via Commons - https://commons.wikimedia.org/wiki/File:Emission_spectrum-H.svg#/media/File:Emission_spectrum-H.svg
- "Fraunhofer lines" by Fraunhofer_lines.jpg: nl:Gebruiker:MaureenVSpectrum-sRGB.svg: PhroodFraunhofer_lines_DE.svg: *Fraunhofer_lines.jpg: Saperaud 19:26, 5. Jul. 2005derivative work: Cepheiden (talk)derivative work: Cepheiden (talk) - Fraunhofer_lines.jpgSpectrum-sRGB.svgFraunhofer_lines_DE.svg. Licensed under Public Domain via Commons - https://commons.wikimedia.org/wiki/File:Fraunhofer_lines.svg#/media/File:Fraunhofer_lines.svg
- http://venables.asu.edu/quant/Dinesh/Bohratom2.html (bohr atom)
- "Energy levels" by SVG: Hazmat2 Original: Rozzychan - This file was derived from: Energylevels.png. Licensed under CC BY-SA 3.0 via Commons - https://commons.wikimedia.org/wiki/File:Energy_levels.svg#/media/File:Energy_levels.svg