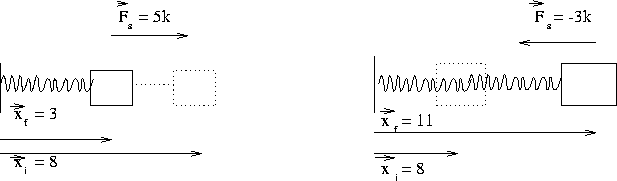Potential Energy of Macroscopic Springs: Difference between revisions
No edit summary |
No edit summary |
||
| Line 21: | Line 21: | ||
#How is it connected to your major? | #How is it connected to your major? | ||
As my major is Chemical Engineering, thermodynamics has many materials in common because of calculating the energy balances toward a reaction. The first law of thermodynamics | As my major is Chemical Engineering, thermodynamics has many materials in common because of calculating the energy balances toward a reaction. The first law of thermodynamics To work out thermodynamic problems you will need to isolate a certain portion of the universe, the system, from the remainder of the universe, the surroundings. | ||
#Is there an interesting industrial application? | #Is there an interesting industrial application? | ||
| Line 37: | Line 36: | ||
== See also == | == See also == | ||
Potential Energy | |||
Ideal Spring | |||
Spring stretch. | |||
===Further reading=== | ===Further reading=== | ||
[[Potential Energy:https://en.wikipedia.org/wiki/Potential_energy]] | |||
==References== | ==References== | ||
[https://en.wikipedia.org/wiki/William_John_Macquorn_Rankine] | |||
[https://en.wikipedia.org/wiki/Spring] | |||
[ | |||
Revision as of 00:57, 6 December 2015
Ideal Spring
The Main Idea
Since real springs have limits as to stretching or compressing, ideal Springs are used for a both real macroscopic spring and for spring-like interatomic bonds.
A Mathematical Model
{Us = 0.5*ks*s^2}
Examples
Connectedness
- How is this topic connected to something that you are interested in?
This is the first law of thermodynamics where every energy related goes around with this law, where energy is neither made or destroyed. It is very interesting how energy is just there and is transformed into other energies such as chemical energy that the food in the student center has will transform into kinetic energy when playing tennis after school.
- How is it connected to your major?
As my major is Chemical Engineering, thermodynamics has many materials in common because of calculating the energy balances toward a reaction. The first law of thermodynamics To work out thermodynamic problems you will need to isolate a certain portion of the universe, the system, from the remainder of the universe, the surroundings.
- Is there an interesting industrial application?
There was an interesting industrial application where we can calculate the energy required by the machine to pump the fluid out.
History
- William Rankinet
- The term potential energy was introduced e, although it has links to Greek philosopher Aristotle's concept of potentiality.
- Scottish engineer and physicist
- links to Greek philosopher Aristotle's concept of potentiality
See also
Potential Energy Ideal Spring Spring stretch.
Further reading
Potential Energy:https://en.wikipedia.org/wiki/Potential_energy