Polarization of an Atom
Claimed and Edited by Julia Woodall Spring 2019
Claimed by Owen Fisher (ofisher3)
Edited by Aniruddha Nadkarni Fall 2016
This page serves to outline and explain the inner workings and hidden mechanisms of the polarization of an atom.
The Main Idea
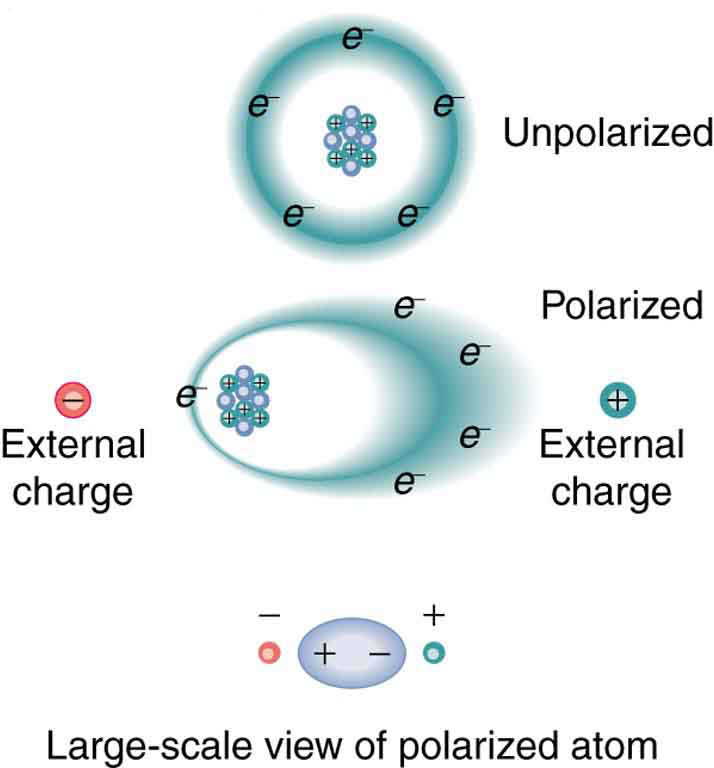
Atoms are basic units of matter, consisting of different charged particles. An atom contains a nucleus made of protons (with +e charge) and neutrons (neutral) which is surrounded by an "electron cloud" defined by a space where electrons (with -e charge) exist. While electrons are attracted to the nucleus and therefore generally remain near the the nucleus, electrons can also be shifted by external charges. These external charges therefore allow electrons to shift so that the electron cloud is no longer centered on the nucleus, and this results in an symmetric distribution of charge allowing the atom to be "polarized".
More specifically, these external charges create apply an electric force on atom constituents. For example, if a positive charge is placed to the left of an atom, an electric field will be created that shifts the electron cloud of the atom towards the positive charge (to the left) and will shift the net positive nucleus away from the charge (to the right) as two objects of the same charge repel each other. In this case, it is now more probable to find an electron to the left of the nucleus, rather than the right.
A Mathematical Model
The following are equations mediating these interactions. First and most importantly, electric fields are the product of field source charge and the distance to the observation location at which you would like to find the electric field value. Coulomb's Law allows us to calculate this for a point charge:
[math]\displaystyle{ {\vec{E} = \frac{1}{4\pi\varepsilon_0}} }[/math] \frac{1}{4\pi\varepsilon_0} [math]\displaystyle{ {\vec{F} = q\vec{E}} }[/math] where F is the force created by the electric field E and the charge of a particle q. This force is what causes the atom to become polarized.
[math]\displaystyle{ {\vec{p} = α\vec{E}} }[/math] for almost all materials, the dipole moment p of the polarized atoms or molecules is directly proportional to the magnitude of the applied electric field E. The constant α is called the "polarizability" of a particular material. Many of these polarizability values have been measured experimentally and can be found in reference volumes.
A Computational Model
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Suppose that you have a negatively charged tape hanging from the desk, and you rub a wooden pencil on a wool sweater and bring it near the tape.
- If the tape swings toward the pencil, does this show that the pencil had been positively by rubbing it on the wool?
Not necessarily. Even if the pencil is uncharged, the charged tape will polarize the and be attracted by the induced dipoles.
- Can a charged object repel a neutral object? Why or why not?
Polarization always brings the unlike-sign charge closer, yielding a net attraction. Repulsion of an induced dipole can't happen. Therefore repulsion is the better test of whether an object is charged.
Middling
- Question Why are charged objects attracted to neutral objects?
The attraction of both positively and negatively charged invisible tape to your hand, and to many other neutral objects, is deeply mysterious. The net charge of a neutral object is zero, so your neutral hand should not make an electric field that could act on a charged tape, nor should your neutral hand experience a force due to the electric field made by a charged tape. Nothing in our statement of the properties of electric interactions allows us to explain this attraction!
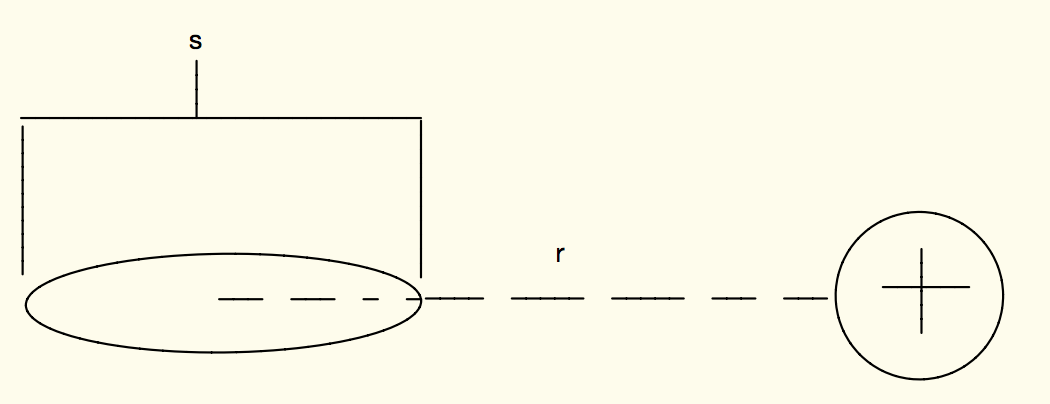
A positive point charge with charge q acts on a an atom as shown below.
What is the electric dipole moment p of the atom?
[math]\displaystyle{ {\vec{p} = α\vec{E}} }[/math]
[math]\displaystyle{ E=\frac{1}{4 \pi \epsilon_0 } \frac{q}{r^2} \hat{r} }[/math]
[math]\displaystyle{ p=\frac{\alpha}{4 \pi \epsilon_0 } \frac{q}{r^2} \hat{r} }[/math]
What is the magnitude and direction of the electric force due to the induced electric field on the point charge? Assume the magnitude of the charge for either end of the dipole is q, and that r is much larger than s.
[math]\displaystyle{ {|{E_{dipole,axis}}|=\frac{1}{4 \pi \epsilon_0 } \frac{2q^2s}{r^3} \hat{r} }[/math]
The [math]\displaystyle{ q^2 }[/math] in this equation comes from the fact that we multiply the Electric field by the charge it is acting on to get the electric force. The direction of this force will be in the negative x direction, since the negative end of the atom would be polarized closer to the positive point charge, and thus the force acting due to the electric field would also be in that direction.
Difficult
Connectedness
How is this topic connected to something that you are interested in?
- Atoms are the composition of all life. Anything can be broken down into atoms and subatomic particles. If we are able to understand atoms we understand the fundamental concepts of all life, and that is pretty interesting in my opinion.
How is it connected to your major?
- Polarization of atoms is not directly related to my major of Mechanical Engineering; however, there are classes I am required to take such as Intro to Physics 2 and Chemistry where the polarization of atoms directly applies.
Is there an interesting industrial application?
- There are many interesting industrial applications of the polarization of atoms over a broad scope of fields.
- The polarization of atoms is what causes something known as van Der Waals forces, which are attractive forces between molecules or atoms that are weaker than traditional covalent or ionic bonds, but still play a fundamental role in the behavior of such particles. These forces are what allow creatures like spiders and geckos to scale walls, as the van Der Waals forces between small hairs for spiders and another adhesive material for geckos interact with the particles of the surface being scaled, and are strong enough to support the animal. Using technology that mimics this behavior, mechanical and materials engineering labs have created basic prototypes for tools that can allow humans to scale walls in the same fashion, which could be applied for construction work, rescue utility, and countless other applications.
Some of these include:
Chemistry
- Checking chirality of organic compounds
- Infrared spectroscopy
Astronomy
- Providing information on sources of radiation and scattering, polarization probes the interstellar magnetic field
- Polarization of cosmic microwave background is being used to study the physics of the early universe
3D Movies
- Images are projected from the projector with multiplexed polarization
- 3D glasses with suitable polarized filters ensure that each eye receives only the intended image
Communication and Radar
- All radio transmitting and receiving antennas are intrinsically polarized-think FM and AM radio
- Vertical polarization is used to radiate a radio signal in all directions, such as those used in mobile phones
- Alternating vertical and horizontal polarization allows satellite communication systems to broadcast two separate transmissions on a single frequency
Material Science Engineering
- the relationship between strain and birefringence motivates the use of polarization in characterizing the distribution of stress and strain in prototypes
Navigation
- Sky polarization was used in the 1950s when navigating near the poles of the Earth's magnetic field when neither the sun nor stars were visible
History
The two scientists accredited with first coming up with the electron cloud model of the atom, on which atom polarization is based upon, are Ernest Rutherford, a New Zealand born British scientist, and Niels Bohr, a Danish physicist. Both of these gentlemen's atom models included the electron cloud. Rutherford released his model in 1911, and Bohr came out with his model shortly thereafter in 1913. Rutherford's model however, suggested that all atoms were unstable. Bohr corrected this by suggesting that the electrons in the atom could only have certain classical motions. Without these two men, we could never have discovered how the polarization of an atom works.
See also
Further reading
- Polarization Functions for First and Second Row Atoms in Gaussian type MO-SCF Calculations by B. Roos and P. Siegbahn
- General Contraction of Gaussian Atomic Orbitals: Core, Valence, Polarization, and Diffuse Basis Sets by Richard C. Raffenetti
- Polarization Propagator Methods in Atomic and Molecular Calculations by Jens Oddershede, Poul Jørgensen, and Danny L. Yeager
- Phase of the Atomic Polarization in High-Order Harmonic Generation by Maciej Lewenstein, Pascal Salières, and Anne L’Huillier
External links
http://www.physicsclassroom.com/class/estatics/Lesson-1/Polarization
http://academics.smcvt.edu/abrizard/EM/dielectric_I.pdf
http://budker.berkeley.edu/papers/pdfs/QBvisualisationPreprint.pdf
http://www.hho4free.com/electrical_polarization.htm
References
https://www.webassign.net/ebooks/mi4/toc.html?page=14.3
http://www.slideshare.net/pabitadhungel321/polarization-and-its-application