Electromagnetic Radiation: Difference between revisions
| Line 121: | Line 121: | ||
[[File:visible_EM_modes.png]] | [[File:visible_EM_modes.png]] | ||
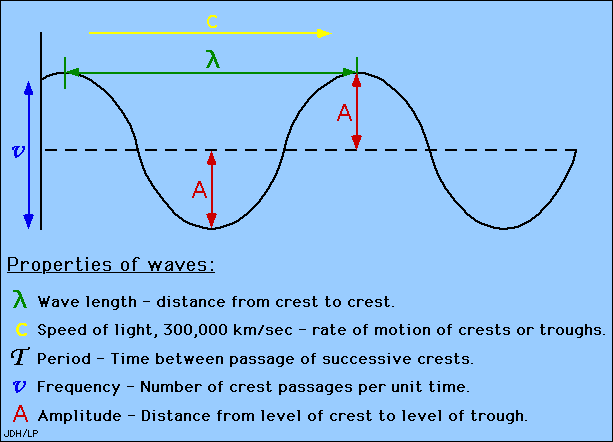
This diagram shows all properties of waves: | |||
[[File:wave_props.gif]] | |||
'''ENERGY FLUX''' | '''ENERGY FLUX''' | ||
Revision as of 21:57, 23 November 2016
Claimed by Carlos Fernandez to edit (Spring 2016) Work on progress by Sungyoung Joo(FALL 2016)
Electromagnetic Radiation
What is a Electromagnetic(EM) Radiation?
Electromagnetic radiation is a form of energy that is all around us and takes many forms, such as radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays.
Before 1873, electricity and magnetism were thought to be two different forces. However, in 1873, Scottish Physicist James Maxwell developed his famous theory of electromagnetism. There are four main electro magnetic interactions according to Maxwell:
- The force of attraction or repulsion between electric charges is inversely proportional to the square of the distance between them
- Magnetic poles come in pairs that attract and repel each other much as electric charges do
- An electric current in a wire produces a magnetic field whose direction depends on the direction of the current
- A moving electric field produces a magnetic field, and vice versa
The four Maxwell's Equations provide a complete description of possible spatial patterns of electric and magnetic field in space.
Other than Maxwell's Four equations, there are general properties of all electromagnetic radiation:
- Electromagnetic radiation can travel through empty space. Most other types of waves must travel through some sort of substance. For example, sound waves need either a gas, solid, or liquid to pass through in order to be heard
- The speed of light is always a constant (3 x 10^8 m/s)
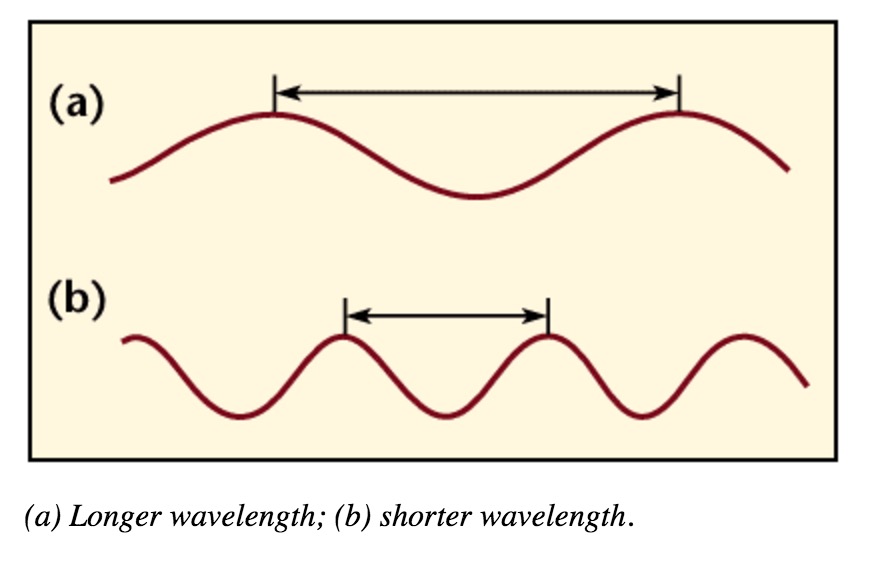
- Wavelengths are measured between the distances of either crests or troughs. It is usually characterized by the Greek symbol λ (gamma).
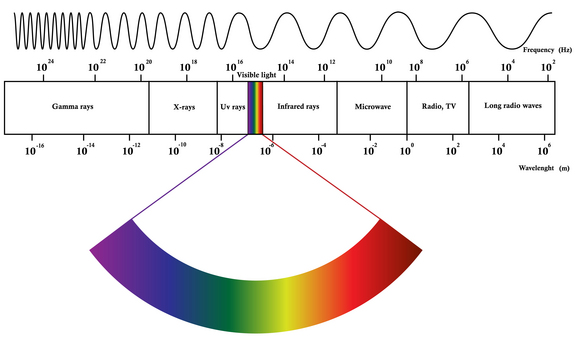
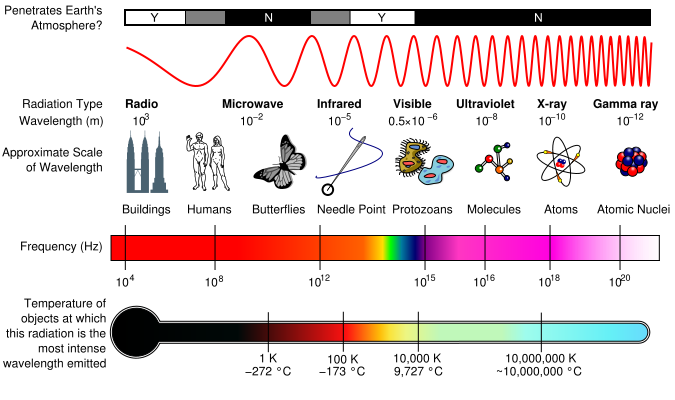
The EM Spectrum
EM spectrum is a span of enormous range of wavelengths and frequencies. The EM spectrum is generally divided into 7 different regions, in order of decreasing wavelength and increasing energy and frequency. It ranges from Gamma rays to Long Radio Waves. Following are the lists of waves:
- Gamma rays
- X-rays
- UV rays
- Visible Light
- Infrared Rays
- Microwave
- Radio, TV
- Long radio waves
Although all these waves do different things, there is one thing in common : They all travel in waves.
Infrared radiation can be released as heat or thermal energy. It can also be bounced back, which is called near infrared because of its similarities with visible light energy. Infrared Radiation is most commonly used in remote sensing as infrared sensors collect thermal energy, providing us with weather conditions.
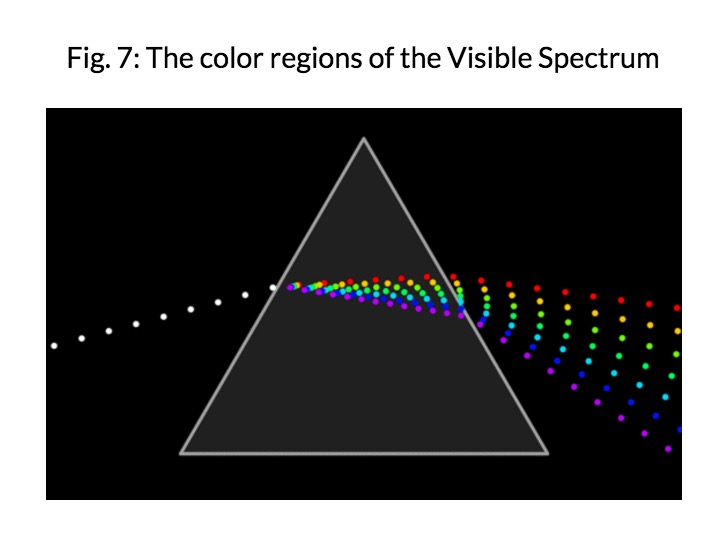
Visible Light is the only part of the electromagnetic spectrum that humans can see with a naked eye. This part of the spectrum includes a range of different colors that all represent a particular wavelength. Rainbows are formed in this way; light passes through matter in which it is absorbed or reflected based on its wavelength. As a result, some colors are reflected more than other, leading to the creation of a rainbow.
Waves and Fields
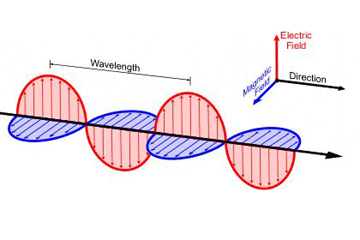
As we learned in class, electric field is produced when an electron is accelerating. Likewise, EM radiation is created when an atomic particle, like an electron, is accelerated by an electric field. The movement like this produces oscillating electric and magnetic fields, which travel at right angles to each other in a bundle of light energy called a photon. Photons travel in a harmonic wave at the fastest speed possible in the universe.
Electromagnetic waves are formed when an electric field couples with a magnetic field. Magnetic and electric fields of an electromagnetic wave are perpendicular to each other and to the direction of the wave.
A wavelength (in m) is the distance between two consecutive peaks of a wave. Frequency is the number of waves that form in a given length of time. A wavelength and frequency are interrelated. A short wavelength indicates that the frequency will be higher because one cycle can pass in a shorter amount of time. Likewise, a longer wavelength has a lower frequency because each cycle takes longer to complete.
A Mathematical Model
The position of the particle is defined by a sine wave:
y = ymaxsin(wt)
Where w is the angular frequency.
Amplitude
Amplitude is the distance from the maximum vertical displacement of the wave to the middle of the wave. The Amplitude of the sinusoidal Wave is the height of the peak in the wave measured from the zero line. This measures the magnitude of oscillation of a particular wave. The Amplitude is important because it tells you the intensity or brightness of a wave in comparison with other waves.
Period
The period of the wave is the time between crests in seconds(s).
T = 2pi/w-----(units of seconds)
Frequency
Frequency is the number of cycles per second, and is expressed as sec-1 or Hertz(Hz). Frequency is directly proportional to energy and can be express as "
E = hv where E is energy, h is Planck's constant ( 6.62607*10^-34J) and v is frequency
f = 1/T
f = w/2pi----(Units Hertz)
Wavelength
Wavelength is the distance between crests in meters. Wavelength is equal to the speed of light times frequency. Longer wavelength waves such as radio waves carry low energy; this is why we can listen to the radio without any harmful consequences. Shorter wavelength waves such as x-rays carry higher energy that can be hazardous to our health.
Wavelength and Frequency
The speed of light is the multiplication of the wavelength and frequency.
c=λν
This diagram shows all properties of waves:
ENERGY FLUX
Is defined by the following equation:
S = (1/u0)*(E x B) in W/m^2
where B = E/c
where c = speed of light
History
Electromagnetic radiation of wavelengths in the early 19th century. The discovery of infrared radiation is ascribed to astronomer William Herschel, who published his results in 1800 before the Royal Society of London. Herschel used a glass Triangular prism (optics)|prism to refract light from the Sun and detected invisible rays that caused heating beyond the red part of the spectrum, through an increase in the temperature recorded with a thermometer. These "calorific rays" were later termed infrared.