Charge Transfer: Difference between revisions
No edit summary |
Robbiemcc7 (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
claimed by | claimed by '''Robinson McCormick fall 2018''' | ||
If a charged conductor comes in contact, or is in close enough proximity, with another conductor, it is possible to transfer this charge to the second conductor. This process is called '''charge transfer'''. | If a charged conductor comes in contact, or is in close enough proximity, with another conductor, it is possible to transfer this charge to the second conductor. This process is called '''charge transfer'''. However, the ''Law of Conservation of Charge'' states that charges cannot be created or destroyed. Charge cannot be created, the presence of a negative charge is merely the effect of an object gaining electrons from another material. Since charge cannot be created or destroyed, and is just the transfer of electrons between materials, the magnitude of the charge transfer between two objects will be equivalent. For however much the charge of one object increases during charge transfer, the other must decrease the same amount. There are multiple ways that charge can be transferred such as through direct contact (ie friction), induction, and conduction. | ||
==Insulators vs Conductors== | ==Insulators vs Conductors== | ||
| Line 10: | Line 10: | ||
On the other hand, electrons are able to flow freely from particle to particle within '''conductors'''. When charge is transferred to a conductor, the charge is distributed evenly across the surface of the object via ''electron movement''. The electrons will be distributed until the repelling force between the excess electrons is minimized. This is the main difference between insulators and conductors: insulators do not have mobile charged particles whereas conductors have mobile charged particles that allow for charge transfer through the free movement of electrons. Examples of insulators include rubber and air and examples of conductors include metals and salt water. | On the other hand, electrons are able to flow freely from particle to particle within '''conductors'''. When charge is transferred to a conductor, the charge is distributed evenly across the surface of the object via ''electron movement''. The electrons will be distributed until the repelling force between the excess electrons is minimized. This is the main difference between insulators and conductors: insulators do not have mobile charged particles whereas conductors have mobile charged particles that allow for charge transfer through the free movement of electrons. Examples of insulators include rubber and air and examples of conductors include metals and salt water. | ||
==Charge by Friction== | |||
Certain objects and materials have a greater attraction to electrons than others. For example, rubber is highly attracted to electrons, whereas fur or hair has a lower attraction. Therefore, if you take a balloon and rub it on your head electrons previously in the atoms of the hair will be pulled to the atoms of the rubber balloon. This creates an electron imbalance which makes the balloon negatively charged and the hair positively charged. This creates the effect where the hair will stand up and be pulled towards the balloon since the opposing charges make the two objects attracted. Likewise, two charged balloons will repel each other. Another example of this is rubbing a glass rod with silk. The glass rod will become positively charged and the silk will become negatively charged; this means that electrons were transferred from the glass rod to the silk, since protons are not removed from the nuclei. Rubbing two objects together is not necessary for charge transfer, but because rubbing creates more points of contact between two objects, it facilitates charge transfer. | |||
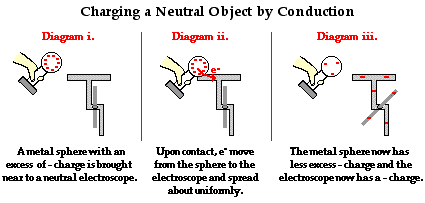
==Transfer Charges by Conduction== | ==Transfer Charges by Conduction== | ||
As shown in the previous section electrons move from one object to another through points of contact, this is especially true among metals. Charging by conduction requires the contact of a charged metal, positive or negative, to a neutral metal. If a negatively charged object touches the neutral metal, the excess electrons will flow through the neutral object. Since electrons repel each other and the negatively charged metal has a buildup of electrons, when given an outlet in the form of the neutral metal a certain number of excess electrons will flow out and spread across the neutral object. This process leaves both metals negatively charged. The same process occurs with positively charged objects touching neutral metals. Additionally, as a note, this process only works between two conductors an insulator cannot undergo conduction. | |||
[[File:Conductiontransfer.gif]] | [[File:Conductiontransfer.gif]] | ||
| Line 19: | Line 22: | ||
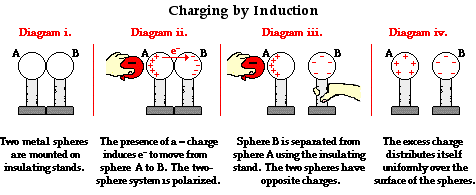
==Transfer Charges by Induction== | ==Transfer Charges by Induction== | ||
Unlike the transfer of charges by conduction, objects | Unlike the transfer of charges by conduction, objects can transfer charges without making contact. When an object is charged, it has an electric field. This electric field will repel or attract electrons in another object. This electron movement is called transfer of charges by induction. A neutral object can be charged by another charged object through a process called '''polarization'''. This is when electrons in the object is repelled or attracted to one side of the object by the charged second object. For example, if a negatively charged sphere is placed near a neutral sphere, the electrons in the neutral sphere will be repelled by the charged sphere. The neutral sphere is now polarized, with one side of it being negatively charged and the other side being positively charged. The negatively charged side of the sphere can be removed through grounding or with a conductor. Once removed, the originally neutral sphere will now be positively charged. Another example of induction is the balloon and black pepper experiment. A balloon can be given a negative charge by rubbing it on hair. When the balloon is placed near grounded black pepper, the black pepper particles will be polarized so that it becomes positively charged on top and will be attracted to the negatively charged balloon. | ||
[[File:Indtransfer.gif]] | [[File:Indtransfer.gif]] | ||
Revision as of 21:46, 25 November 2018
claimed by Robinson McCormick fall 2018
If a charged conductor comes in contact, or is in close enough proximity, with another conductor, it is possible to transfer this charge to the second conductor. This process is called charge transfer. However, the Law of Conservation of Charge states that charges cannot be created or destroyed. Charge cannot be created, the presence of a negative charge is merely the effect of an object gaining electrons from another material. Since charge cannot be created or destroyed, and is just the transfer of electrons between materials, the magnitude of the charge transfer between two objects will be equivalent. For however much the charge of one object increases during charge transfer, the other must decrease the same amount. There are multiple ways that charge can be transferred such as through direct contact (ie friction), induction, and conduction.
Insulators vs Conductors
In an insulator, electrons are bounded tightly to atoms, which prevents charged particles from moving through the material. If charge is transferred to an insulator at a given location, the charge will remain at the location that the transfer occurred.
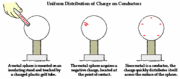
On the other hand, electrons are able to flow freely from particle to particle within conductors. When charge is transferred to a conductor, the charge is distributed evenly across the surface of the object via electron movement. The electrons will be distributed until the repelling force between the excess electrons is minimized. This is the main difference between insulators and conductors: insulators do not have mobile charged particles whereas conductors have mobile charged particles that allow for charge transfer through the free movement of electrons. Examples of insulators include rubber and air and examples of conductors include metals and salt water.
Charge by Friction
Certain objects and materials have a greater attraction to electrons than others. For example, rubber is highly attracted to electrons, whereas fur or hair has a lower attraction. Therefore, if you take a balloon and rub it on your head electrons previously in the atoms of the hair will be pulled to the atoms of the rubber balloon. This creates an electron imbalance which makes the balloon negatively charged and the hair positively charged. This creates the effect where the hair will stand up and be pulled towards the balloon since the opposing charges make the two objects attracted. Likewise, two charged balloons will repel each other. Another example of this is rubbing a glass rod with silk. The glass rod will become positively charged and the silk will become negatively charged; this means that electrons were transferred from the glass rod to the silk, since protons are not removed from the nuclei. Rubbing two objects together is not necessary for charge transfer, but because rubbing creates more points of contact between two objects, it facilitates charge transfer.
Transfer Charges by Conduction
As shown in the previous section electrons move from one object to another through points of contact, this is especially true among metals. Charging by conduction requires the contact of a charged metal, positive or negative, to a neutral metal. If a negatively charged object touches the neutral metal, the excess electrons will flow through the neutral object. Since electrons repel each other and the negatively charged metal has a buildup of electrons, when given an outlet in the form of the neutral metal a certain number of excess electrons will flow out and spread across the neutral object. This process leaves both metals negatively charged. The same process occurs with positively charged objects touching neutral metals. Additionally, as a note, this process only works between two conductors an insulator cannot undergo conduction.
Transfer Charges by Induction
Unlike the transfer of charges by conduction, objects can transfer charges without making contact. When an object is charged, it has an electric field. This electric field will repel or attract electrons in another object. This electron movement is called transfer of charges by induction. A neutral object can be charged by another charged object through a process called polarization. This is when electrons in the object is repelled or attracted to one side of the object by the charged second object. For example, if a negatively charged sphere is placed near a neutral sphere, the electrons in the neutral sphere will be repelled by the charged sphere. The neutral sphere is now polarized, with one side of it being negatively charged and the other side being positively charged. The negatively charged side of the sphere can be removed through grounding or with a conductor. Once removed, the originally neutral sphere will now be positively charged. Another example of induction is the balloon and black pepper experiment. A balloon can be given a negative charge by rubbing it on hair. When the balloon is placed near grounded black pepper, the black pepper particles will be polarized so that it becomes positively charged on top and will be attracted to the negatively charged balloon.
See also
http://www.physicsbook.gatech.edu/Charge_Motion_in_Metals
http://www.physicsbook.gatech.edu/Polarization
References
Internet resources on this topic:
http://www.physicsclassroom.com/class/estatics/Lesson-1/Conductors-and-Insulators
http://www.physicsclassroom.com/class/estatics/Lesson-1/Charge-Interactions
Chabay, Ruth W., Bruce Sherwood. Matter and Interactions, Volume II: Electric and Magnetic Interactions, 4th Edition. Wiley, 19/2015. VitalBook file.