Change of State
Created and Edited by Maite Marin-Mera (Spring 2017)
Short Description of Topic
The Main Idea
All matter can move from one state to another under the right conditions. Depending on the properties of the matter changing states may require extreme temperature or pressure however it can be done. There are five different states of matter; gas, liquid, solid, plasma, and Bose-Einstein condensate.[1] The main idea of this wiki page is to discuss the properties of matter as it transitions between different states and how this relates to energy transfer.
An Overview
All matter can transition between the states dependent on its intrinsic properties. During these transitions there is a large change on the microscopic and macroscopic level of the matter. There is also typically a transfer of energy either into of from the matter undergoing the change.
Solid/Liquid
A very common phase change is between liquid and solids. This change of state is referred to as freezing (liquid to solid) or melting/fusion (solid to liquid).
So what is going on a microscopic level? In a solid the atoms and molecules are packed tightly together. This tightly packed arrangement does not allow for much movement between the particles. Therefore a solid has low kinetic energy. In the liquid phase the particles of a substance have more kinetic energy that those in a solid. The atoms and molecules have more movement resulting in a higher kinetic energy. In the change of state from solid to liquid there is energy required to overcome the binding forces that maintain its solid structure. This energy is called the heat of fusion. In the change of state from liquid to solid energy is given off. The energy given off by this transition is the same amount as the energy required to freeze the matter.
Liquid/Gas
A very common phase change is between liquid and gases. This change of state is referred to as vaporization/boiling (liquid to gas) or condensation (gas to liquid).
So what is going on a microscopic level? In a liquid the atoms and molecules are moving less than they would in the gas state. Therefore the gaseous state has a higher kinetic energy than the liquid state. This is due to the fact that atoms and molecules in liquids are still packed together more closely than the atoms and molecules in a gas. In the change of state from liquid to gas there is energy required to overcome the bonds between the more closely packed atoms and molecules. This energy is called the heat of vaporization. In the change of state from gas to liquid energy is given off by the transition. This energy is equal in magnitude to the energy required to transition from liquid to gas.
Other States
Transitions into the two lesser known states are much harder to analyze and understand. As these states are only present under extreme and unique conditions they will not be discussed in depth. There are links below for further reading on these topics.
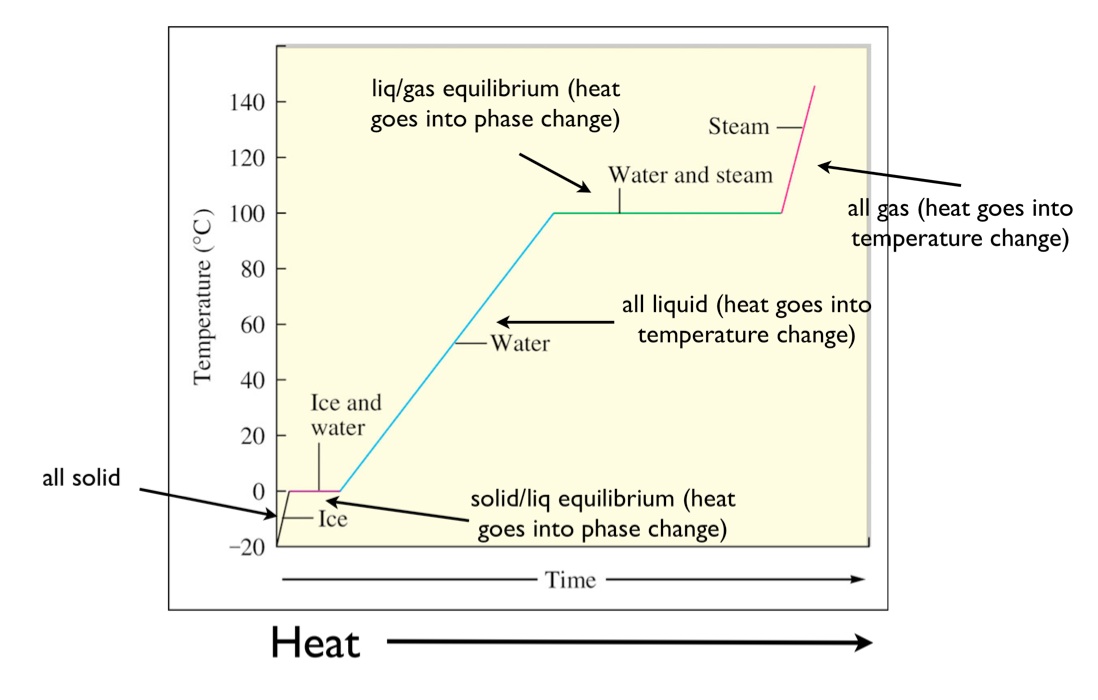
Heating Curve
A common form of depicting the temperature at which a substance changes states and also how much heat is required to change state is a heating curve. The heating curve for water is shown below.
Note that at the point in which the liquid changes state there is no change in temperature. This is because the heat applied is going towards changing the bond structure of the matter and not towards heating the substance.
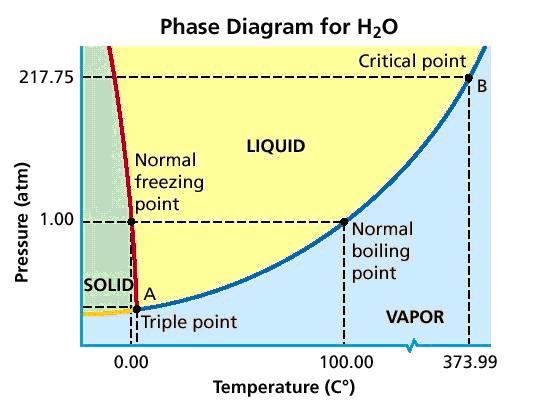
Phase Diagram
A common form of depicting the relationship between pressure, temperature, and the state of a substance is in a phase diagram. The phase diagram for water is shown below.
Note that there is the boundary lines which denote and which temperature and pressure changes in state will occur.
A Mathematical Model
There are multiple different ways of modeling changes in state mathematically. The most common form is using the equations
Q=mcΔT and Q=mH(Heat of Fusion/Vaporization)
m= mass of substance n=moles of substance c= specific heat of substance
As shown in the heating curve above these equations are used interchangeably depending on what the final and initial temperature the substance will be at.
A Computational Model
Examples
Simple
1) What is the heat needed to melt 3g of ice?
Q=mH Heat of Fusion for water= 334 J/g°C [2] Q= 3(334) = 1002 J
Middling
1) Calculate the heat needed/given off when 20g of water at 52°C is cooled to 27°C
Q=mcΔT Specific Heat of Water= 4.184J/g°C [3]
Q= 20(4.184)(27-52)= -2092 J This means that 2092 J are given off by this process.
Difficult
1) Calculate the heat needed to heat 30 g of water from -1°C to 78°C
You have to break this problem into multiple different steps.
First you calculate the Q from the change in temperature to get it to 0°C. Q1=mcΔT Q1=30(4.184)(0-(-1))= 125.52J
Then you calculate the Q from the phase change Q2=mH Q2= 30(334) = 10020 J
First you calculate the Q from the change in temperature to get it to 78°C. Q3=mcΔT Q3=30(4.184)(78-0)= 9790.56J
Then you add all the Q's together Q1 +Q2 +Q3= 125.52+10020+9790.56= 19936.08
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic