Change of State
Edited by William Pardo (Fall 2025)
The Main Idea
All matter can move from one state to another under the right conditions. Depending on the properties of the matter changing states may require extreme temperature or pressure however it can be done. There are five different states of matter; gas, liquid, solid, plasma, and Bose-Einstein condensate.[1] The main idea of this wiki page is to discuss the properties of matter as it transitions between different states and how this relates to energy transfer.
An Overview
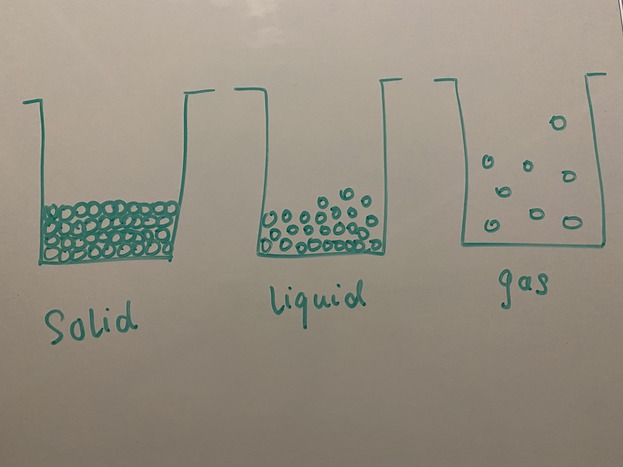
All matter can transition between the states dependent on its intrinsic properties. During these transitions there is a large change on the microscopic and macroscopic level of the matter. There is also typically a transfer of energy between the matter and its surroundings during such a transition - either the matter absorbs energy, or it releases energy to transition between states.
Solid/Liquid
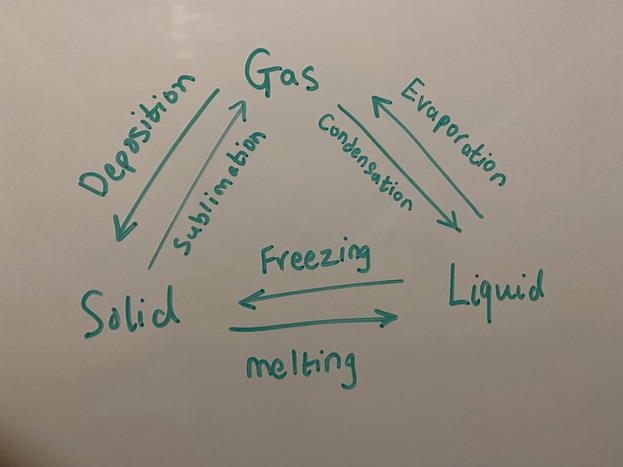
A very common phase change is between liquid and solids. When a liquid is converted to a solid, this change of state is referred to as freezing, and it is an exothermic reaction i.e. it releases heat, warming up its surroundings. Conversely, when a solid is converted to a liquid, this change of state is referred to as melting/liquefaction and it is an endothermic reaction i.e. it absorbs heat from the surroundings, making them cooler.
So what is going on a microscopic level?
In a solid the atoms and molecules are packed tightly together. This tightly packed arrangement does not allow for much movement between the particles. At a given temperature the kinetic energy is determined by only the temperature, not the state. More heat generally means more movement in the molecules. What changes in comparison between a solid and a liquid (for example) is the particle arrangement and the potential energy. During melting at a constant temperature such as at melting point, the energy added to the system does not increase the kinetic energy, but instead only affects potential energy.
In the change of state from solid to liquid there is energy required to overcome the binding forces that maintain its solid structure. This energy is called the heat of fusion. In the change of state from liquid to solid energy is given off. The energy given off by this transition is the same amount as the energy required to freeze the matter.
Liquid/Gas
A very common phase change is between liquid and gases. This change of state is referred to as vaporization/boiling (liquid to gas) or condensation (gas to liquid).
So what is going on a microscopic level?
In a liquid the atoms and molecules are moving less than they would in the gas state. Therefore the gaseous state has a higher kinetic energy than the liquid state. This is due to the fact that atoms and molecules in liquids are still packed together more closely than the atoms and molecules in a gas. In the change of state from liquid to gas there is energy required to overcome the bonds between the more closely packed atoms and molecules. This energy is called the heat of vaporization. Note that in this process the additional heat is contributing to the system's potential energy as it overcomes the bonds between molecules. In the change of state from gas to liquid energy is given off by the transition. This energy is equal in magnitude to the energy required to transition from liquid to gas.
Solid/ Gas
Sublimation occurs when a substance goes from a solid state directly to a gaseous state, without passing through the liquid state. This process happens when the temperature and pressure conditions are such that the vapor pressure of the solid substance exceeds the ambient pressure. As the solid is heated, its vapor pressure increases until it equals the ambient pressure, at which point the substance begins to sublimate and turn into a gas. Examples of substances that can undergo sublimation include dry ice (solid carbon dioxide), mothballs (naphthalene), and frozen air fresheners (para-dichlorobenzene).
Deposition, on the other hand, is the reverse process of sublimation, where a gas transforms directly into a solid, without passing through the liquid phase. This process occurs when a gas is cooled to a temperature below its sublimation point, at which point it starts to deposit onto a solid surface. Examples of deposition include the formation of frost on a cold surface, the formation of snow from water vapor in the atmosphere, and the deposition of solid carbon dioxide (also known as "dry ice") from gaseous carbon dioxide.
Both sublimation and deposition are important physical processes that play a role in many natural phenomena and industrial applications. For example, sublimation is used in freeze-drying of foods and medicines, while deposition is involved in the formation of snowflakes and other forms of precipitation.
Phase Change Diagram
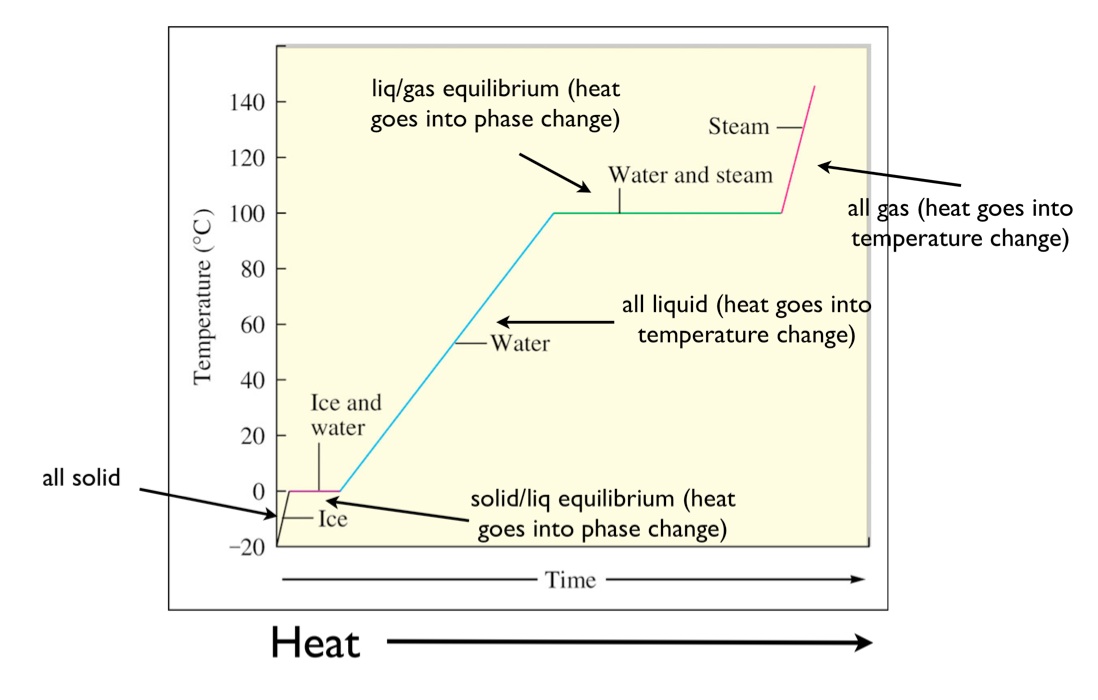
Heating Curve
A common form of depicting the temperature at which a substance changes states and also how much heat is required to change state is a heating curve. The heating curve for water is shown below.
Note that at the point in which the liquid changes state there is no change in temperature. This is because the heat applied is going towards changing the bond structure of the matter and not towards heating the substance.
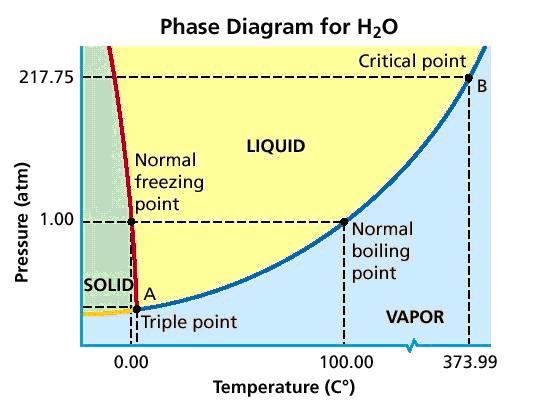
Phase Diagram
A common form of depicting the relationship between pressure, temperature, and the state of a substance is in a phase diagram. The phase diagram for water is shown below.
Note that there is the boundary lines which denote and which temperature and pressure changes in state will occur.
The triple points is the temperature and pressure at which the substance exists in all three phases of matter in equilibrium. The critical point denotes the temperature and pressure beyond which the liquid and gas phases become indistinguishable. The substance at this point is a supercritical fluid, which combines the traits of a gas and a liquid. Supercritical fluids have industry applications such as extractions: deodorizing, seed oil extraction, decaffeination, etc.[2]
A Mathematical Model
There are multiple different ways of modeling changes in state mathematically. The most common form is using the equations
[math]\displaystyle{ Q = m \cdot c \cdot \Delta T }[/math] and [math]\displaystyle{ Q = m \cdot H }[/math] (Heat of Fusion/Vaporization)
m = mass of substance n = moles of substance c = specific heat of substance
As shown in the heating curve above, these equations are used interchangeably depending on what the final and initial temperature the substance will be at.
However, this model is to be used with the caveat that it only works when the matter is in a fixed state i.e. it cannot be applied during state changes such as freezing/ melting and evaporation/ condensation. Instead, the idea of "latent heat" can be used during the change of state.
Latent heat refers to the amount of heat energy required or released during a phase change of a substance, such as melting, freezing, vaporization, or condensation. This energy is used to break or form the intermolecular bonds between the particles of the substance, rather than to increase or decrease the temperature of the substance.
The amount of latent heat involved in a phase change depends on the specific substance and the conditions under which the phase change occurs. The equations used to compute latent heat can vary depending on the specific context, but here are some common ones:
Latent heat of fusion (melting or freezing): The amount of heat energy required to change the state of a substance from a solid to a liquid or vice versa at constant temperature is given by the equation: Q = mL
where Q is the amount of heat energy required or released, m is the mass of the substance, and L is the latent heat of fusion for the substance.
Latent heat of vaporization (boiling or condensation): The amount of heat energy required to change the state of a substance from a liquid to a gas or vice versa at constant temperature is given by the equation: Q = mL
where Q is the amount of heat energy required or released, m is the mass of the substance, and L is the latent heat of vaporization for the substance.
These equations assume that the phase change occurs at constant temperature, which is often the case under normal conditions. However, if the temperature is changing during the phase change, additional equations and considerations may be necessary to accurately calculate the latent heat involved.
Glowscript Code
The following Glowscript code creates a simple system of particles that can exist in three different states: solid, liquid, or gas. The particles are represented by spheres, and their behavior is determined by the current state of the system. The simulation loop updates the positions and velocities of the particles based on the current state, and checks for state transitions based on the positions of the particles.
Note that this is a very basic example, and there are many ways to expand on this code to create more complex simulations of states of matter.
Here is a dynamic version that shows the movement of particles with arbitrary units: [4]
A Computational Model
The concept of state change is a complex and interesting idea that is fundamental to a specialized field of physics known as statistical mechanics. In this field, the central model that describes a general mechanism of state change on the particle level is the Ising Model which utilizes Monte Carlo sampling of particles at certain temperatures in a distribution and assigns these particles a spin of either +1 or -1. The Ising Model is a simple, yet descriptive, simulation of energy state configurations in an “n by n” lattice of spins, with each “spin” being pointed in upwards (+1) or downwards (-1). The direction of the spin indicates a unique energy state of that spin.
Below is a sample simulation created in MATLAB that plots energy dependencies on normalized temperature. To run the simulation, you will need to download the files via the link provided below containing all source code. You will also need MATLAB in order to run the simulation. Make sure to only run the final_program.m file! It relies on the multiple helper functions that are associated with the Ising Model program (i.e. there is no need to run the other helper functions individually. ONLY run the final_program.m file!).
Source: MATLAB (MathWorks)
If you run the simulation, the output should look similar to this plot:
While the output will be next to identical, there are actually slight differences in the actual values of temperature and energy of the species simulated. This is because Monte Carlo sampling was used, so each run is fed with randomly selected spins/temperatures and thus is independent of any other run.
Examples
Simple
1) What is the heat needed to melt 3g of ice?
[math]\displaystyle{ Q = m \cdot H }[/math] Heat of Fusion for water= 334 J/g°C [6] Q= 3(334) = 1002 J
Middling
1) Calculate the heat needed/given off when 20g of water at 52°C is cooled to 27°C
Q=mcΔT Specific Heat of Water= 4.184J/g°C [7]
Q= 20(4.184)(27-52)= -2092 J This means that 2092 J are given off by this process.
Difficult
1) Calculate the heat needed to heat 30 g of water from -1°C to 78°C
You have to break this problem into multiple different steps.
First you calculate the Q from the change in temperature to get it to 0°C. Q1=mcΔT Q1=30(4.184)(0-(-1))= 125.52J
Then you calculate the Q from the phase change Q2=mH Q2= 30(334) = 10020 J
First you calculate the Q from the change in temperature to get it to 78°C. Q3=mcΔT Q3=30(4.184)(78-0)= 9790.56J
Then you add all the Q's together Q1 +Q2 +Q3= 125.52+10020+9790.56= 19936.08
Connectedness
This topic is related to all aspects of everyday life, phase changes are present in multiple different engineering processes. It is important to know where the energy is flowing in these changes of state in order to maximize efficiency. This is applicable to my major as a Biomedical Engineer because there are multiple processes which have changes of state and in order to keep a system balanced there has to be an influx or outflow of energy.
Other States
Here is some useful information on the two states of matter not discussed above, namely plasma and the Bose-Einstein condensate. Plasma: It is a highly ionized gas that contains a significant number of free electrons and positive ions.
Plasma can be created by heating a gas to high temperatures, applying an electric field to a gas, or exposing a gas to intense radiation. This causes some of the gas particles to become ionized, meaning they lose or gain electrons, and become highly charged.
Plasma has some unique properties that distinguish it from the other states of matter. For example, it can conduct electricity and generate magnetic fields, making it useful for applications such as fusion reactors, plasma cutting, and plasma TVs.
Plasma is also the most common state of matter in the universe, as it makes up over 99% of visible matter, including stars and galaxies.
Bose-Einstein Condensate: It is a state of matter that occurs at extremely low temperatures, close to absolute zero (-273.15°C or 0 Kelvin). It is named after Satyendra Nath Bose and Albert Einstein, who first predicted its existence in 1924-1925.
BEC is a state of matter in which a group of bosons (particles that follow Bose-Einstein statistics) collapse into the same quantum state, forming a single macroscopic entity. This means that all the atoms making up the BEC behave as a single entity, rather than as individual atoms.
BEC was first observed experimentally in 1995, when researchers at the University of Colorado and the National Institute of Standards and Technology were able to cool a gas of rubidium atoms to near absolute zero, causing them to collapse into a single quantum state.
BEC has some unique properties that distinguish it from other states of matter. For example, it exhibits wave-like properties and can form interference patterns, similar to the behavior of light waves. It is also incredibly sensitive to external stimuli, such as magnetic and electric fields, making it useful for precision measurements and the development of new technologies such as atom lasers.
History
Changes of states of matter have been studied from the very beginning of the studies of science. From cooking to food and beverage preservation to the hydrological cycle, change of state is a pivotal concept in physics, especially when considering its implications for many related fields. The relation of state change to temperature, or average kinetic energy, is an important one with many historical examples. For instance, the failure of NASA managers to consider the temperature limits of the rubber O-rings on the solid rocket boosters when covered with water in sub-freezing temperatures led to the escape of super-heated gas that inevitably ignited the external tank, leading to the death of all seven astronauts on board the Space Shuttle Challenger. Although a fairly simple concept, the applications of the science behind state change go far beyond the surface and, in many cases, are crucial to the success of projects such as the space shuttle program, one that had to deal with environments (like entry to space/LEO) where state change was common, frequent, and hard to control/adapt to.
Further reading
More on a mathematical model http://www.math.utk.edu/~vasili/475/Handouts/3.PhChgbk.1+title.pdf
External links
https://ch301.cm.utexas.edu/section2.php?target=thermo/enthalpy/heat-curves.html
https://craigssenseofwonder.wordpress.com/tag/phase-diagram/
http://www.chem4kids.com/files/matter_changes.html
http://www.livescience.com/46506-states-of-matter.html