Particle in a 1-Dimensional box
claimed by Eathan 4/14/2022
The time-independent Schrodinger Equation is a partial differential equation whose solutions describe the wave function of a quantum system. It is given in the following form. [math]\displaystyle{ -\frac{\hbar^2}{2m}\frac{\partial^2\psi(x)}{\partial x^2} + V(x)\psi(x)= E \psi(x) }[/math]
where
[math]\displaystyle{ \hbar }[/math] is the reduced Planck constant
[math]\displaystyle{ m }[/math] is the mass of the particle
[math]\displaystyle{ \psi(x) }[/math] is the wave function
[math]\displaystyle{ V(x) }[/math] is the potential of the system
[math]\displaystyle{ E }[/math] is the energy of the system
Main Idea
We can solve the Schrodinger Equation by considering its boundary conditions and applying continuity to them. We will showcase 2 cases of the particle in a 1-Dimensional box: an infinite well and a semi-infinite one.
Infinite Potential (Mathematical Model)
We will first consider the following scenario:
The particle in a 1-Dimensional box is a quantum system in which a particle is bounded in a well with infinite energy at the barrier. Since the barriers of the well are infinite, there should be a 0% chance of finding the particle outside the well. We will apply this boundary condition later as we derive the solution to the particle in a 1-Dimensional box
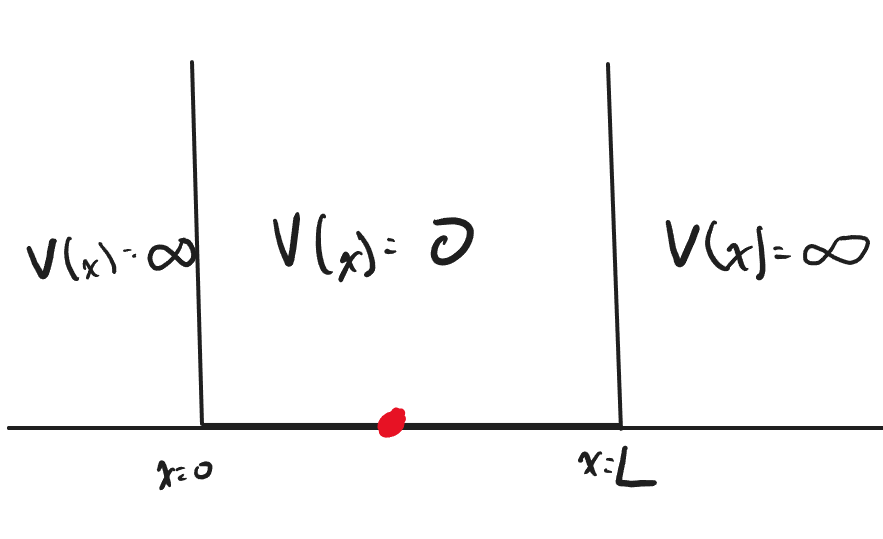
Imagine the quantum system shown above, where the particle is bounded by infinite potential energy at [math]\displaystyle{ x = 0 }[/math] and [math]\displaystyle{ x = L }[/math]. The system also has 0 potential energy within the boundaries. We will use these as our boundary conditions to solve for the wave function [math]\displaystyle{ \psi(x) }[/math] below.
We will begin to solve the Schrodinger equation with the boundary conditions as shown above. From the points [math]\displaystyle{ x = 0 }[/math] to [math]\displaystyle{ x = L }[/math], there is 0 potential energy. Using the time-independent Schrodinger Equation with [math]\displaystyle{ V(x) = 0 }[/math], we arrive at the following Schrodinger Equation: [math]\displaystyle{ -\frac{\hbar^2}{2m}\frac{\partial^2\psi(x)}{\partial x^2}= E \psi(x) }[/math]
Isolating the second partial derivative term gives us the following: [math]\displaystyle{ \frac{\partial^2\psi(x)}{\partial x^2}= -\frac{2mE}{\hbar^2}\psi(x) }[/math]
To simplify this, let us define the constant k as the following: [math]\displaystyle{ k = \frac{\sqrt{2mE}}{\hbar} }[/math]
Rewriting the Schrodinger Equation with the constant k gives us [math]\displaystyle{ \frac{\partial^2\psi(x)}{\partial x^2} + k^2\psi(x) = 0 }[/math]
The general solution to this partial differential equation is very well known and is given here: [math]\displaystyle{ \psi(x) = Asin(kx) + Bcos(kx) }[/math]
We will now begin to solve for the constants A and B. Recall that we have imposed the boundary conditions of the probability of finding the particle to be 0 anywhere outside of [math]\displaystyle{ x = 0 }[/math] to [math]\displaystyle{ x = L }[/math]. This means that the wave function has to be continuous at the boundary.
First, consider the point [math]\displaystyle{ x = 0 }[/math]. Here, [math]\displaystyle{ \psi(0) = 0 }[/math]. For this to be true, we need to let B = 0. This is because [math]\displaystyle{ sin(0) = 0 }[/math], but [math]\displaystyle{ cos(0) = 1 }[/math]. If the [math]\displaystyle{ Bcos(kx) }[/math] term exists in our [math]\displaystyle{ \psi(x) }[/math], then our wave function would not be cointinuous at the point [math]\displaystyle{ x = 0 }[/math].
Next, consider the point [math]\displaystyle{ x = L }[/math]. Here, [math]\displaystyle{ \psi(L) = 0 }[/math]. For this to be true, [math]\displaystyle{ sin(kL) = 0 }[/math] when [math]\displaystyle{ ka = n\pi }[/math]. This is due to the nature of the sin function itself. This means that we can derive the following expression for k:
[math]\displaystyle{ k = \frac{n\pi}{L} }[/math]
Since we are only considering the case where the potentials are infinite at the barriers, we do not need to check for the derivatives of the wave function to be 0. If this were not an infinite potential well, then we would also have to take derivates of the wave function at each point and apply the boundary conditions to allow them to equal 0 as well.
Lastly, we will consider a normalization condition to solve for the constant A. If there is a 0% chance of finding the particle outside the well, then that must be there is a 100% chance of finding the particle somewhere within the well. This condition is expressed mathematically as
[math]\displaystyle{ \int_{0}^{L}\psi(x)^*\psi(x)dx=1 }[/math]
Since [math]\displaystyle{ \psi(x) }[/math] is a real function, we can rewrite the integrand as [math]\displaystyle{ \psi(x)^2 }[/math]. We can then solve for A by doing the following steps:
[math]\displaystyle{ \int_{0}^{L}\psi(x)^2dx=1 }[/math]
[math]\displaystyle{ A^2\int_{0}^{L}sin^2(kx)dx=1 }[/math]
Use the following identity: [math]\displaystyle{ sin^2(\theta) = \frac{1-cos(2\theta)}{2} }[/math]
[math]\displaystyle{ A^2\int_{0}^{L}\frac{1-cos(2\theta)}{2}dx=1 }[/math]
[math]\displaystyle{ A^2(\frac{L}{2} - \frac{sin(2kL}{4k}) = 1 }[/math]
Recall [math]\displaystyle{ kL = n\pi }[/math]
This means that [math]\displaystyle{ \frac{sin(2n\pi)}{4k} = 0 }[/math]
Therefore [math]\displaystyle{ A = \sqrt{\frac{2}{L}} }[/math]
Putting it all together, we arrive at the following wave equation: [math]\displaystyle{ \psi(x) = \sqrt{\frac{2}{a}}sin(\frac{n\pi}{a}x) }[/math]
Each associated wave function has corresponding energy to it. This can be solved by recalling our original definition of k.
[math]\displaystyle{ k^2 = \frac{2mE}{\hbar^2} }[/math]
[math]\displaystyle{ E = \frac{\hbar^2k^2}{2m} = \frac{\hbar^2n^2\pi^2}{2mL^2} }[/math]
We have now solved the particle in a 1-Dimensional box given our initial conditions mathematically!
Infinite Potential (Computational Model)
Here, we can visualize the wave function and its associated probability density for various quantum levels. As you can see, the wave function changes depending on the quantum number and showcases interesting properties regarding its probability density. For quantum numbers greater than 1, there are places in which you are unable to find the particle within the boundary of the well, despite there being 0 potential energy!
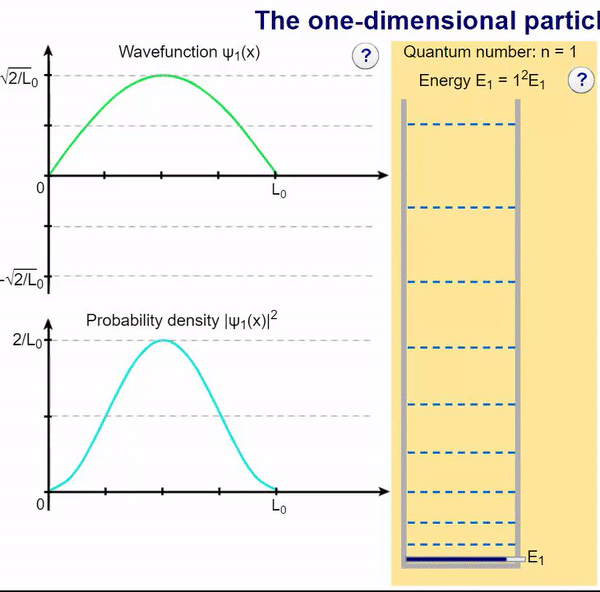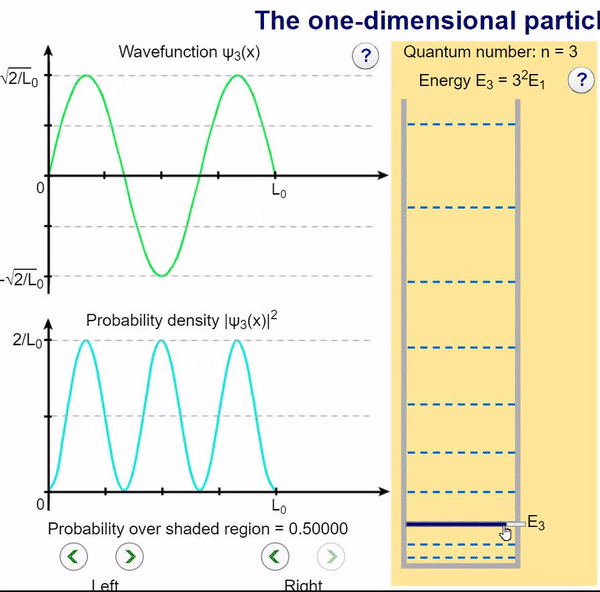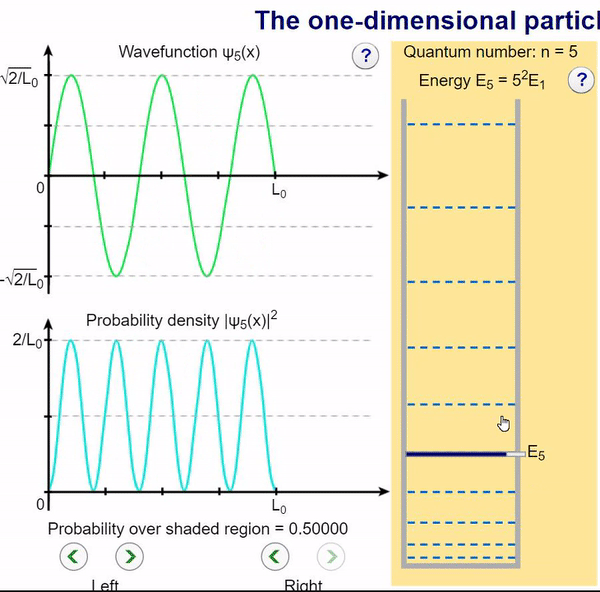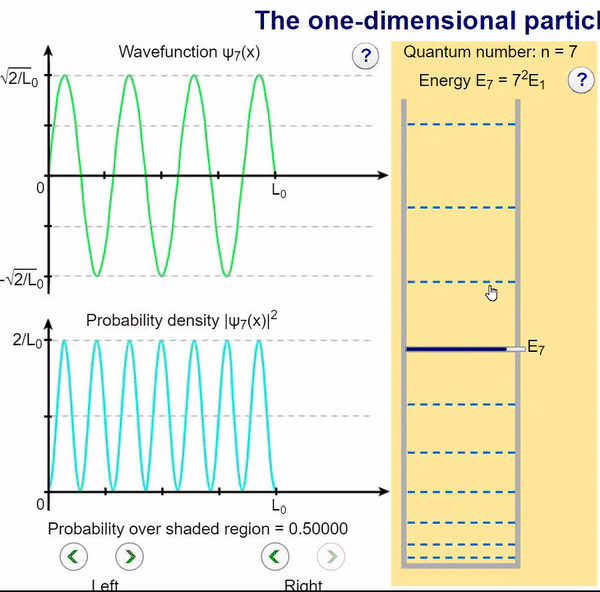
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
This section contains the the references you used while writing this page