Electric Polarization: Difference between revisions
| Line 10: | Line 10: | ||
Conductors are materials that allow electric charge to pass through it since it contains freely moving charged particles. When an electric field is applied to a conductor, electrons are transferred across the surface of the object and it becomes polarized. | Conductors are materials that allow electric charge to pass through it since it contains freely moving charged particles. When an electric field is applied to a conductor, electrons are transferred across the surface of the object and it becomes polarized. | ||
An insulator is a material that does not conduct very much electric charge because all the electrons are rigidly bound to the atoms or molecules; they do not permit the free flow of electrons. When an insulator is subjected to an electric field, individual atoms or molecules are polarized rather than the whole object. | An insulator is a material that does not conduct very much electric charge because all the electrons are rigidly bound to the atoms or molecules; they do not permit the free flow of electrons. When an insulator is subjected to an electric field, individual atoms or molecules are polarized rather than the whole object. | ||
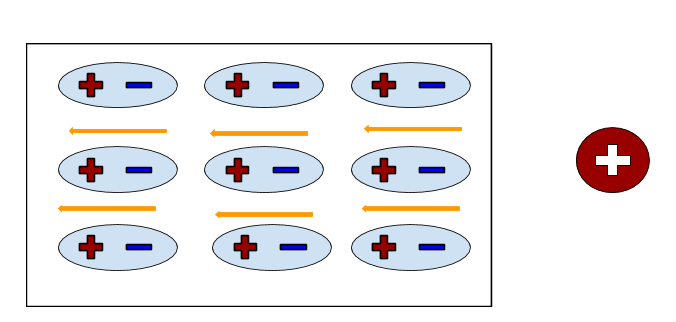
An example of a polarized insulator: | An example of a polarized insulator: | ||
[[File:Screen_Shot_2016-11-27_at_10.52.28_PM.png]] | [[File:Screen_Shot_2016-11-27_at_10.52.28_PM.png]] | ||
Revision as of 22:59, 27 November 2016
Claimed Edit by Eleanor Thomas Fall 2016
The Main Idea
Polarization, used broadly, is the act of dividing into opposites. Electric polarization is the process of separating opposite charges inside an object. This occurs when an electric field, let's say created by a charged object A, induces the electrons to move in object B. This electron movement causes one portion of object B to have an excess negative charge and the other to have an excess positive charge. Object B could be a neutral object with a net charge of zero, but can still be polarized and attracted to object A. If A were positively charged, the electrons in object B would be attracted to the side closest to A (since opposite charges attract) which would create an induced dipole. This dipole is not permanent; if object A were to be removed, B would return to its neutral state.
Cause of Polarization
The cause of polarization can be found when the structure of an atom is closely examined. Atoms have a positively charged nucleus consisting of protons and neutrons tightly clumped together. Surrounding this nucleus is a negatively charged electron cloud which is not rigidly connected to the nucleus. Since the negative electron cloud does not have to be centered at its corresponding positive nucleus, if an electric field is applied on the atom, the two opposite charges can move relative to one another. This applied electric field induced the polarization of atoms.
Conductors and Insulators
Conductors are materials that allow electric charge to pass through it since it contains freely moving charged particles. When an electric field is applied to a conductor, electrons are transferred across the surface of the object and it becomes polarized. An insulator is a material that does not conduct very much electric charge because all the electrons are rigidly bound to the atoms or molecules; they do not permit the free flow of electrons. When an insulator is subjected to an electric field, individual atoms or molecules are polarized rather than the whole object.
An example of a polarized insulator:
Misconception
Neutral objects (with a net charge of zero) CAN be attracted to charged ones because induced dipoles are formed and create an electric field at the location of the object. However, repulsion of an induced object cannot happen as it always brings unlike signs closer.
Mathematical Model
The amount of induced polarization is directly proportional to the magnitude of the applied electric field. Induced Polarization : [math]\displaystyle{ \vec{P} = \alpha \vec{E} }[/math] "P" is the dipole moment of polarized atoms, "α" is the polarizability of a particular material, and "E" is the applied electric field.
Examples
Simple
Question: An electric field is applied to a neutral object by a charged particle near it. After the neutral object is polarized, the particle is removed. What is the charge of the object?
Answer: The object is neutral once again. Polarization is induced by the charged particle; therefore, it is not permanent.
Middling
Question: A balloon is rubbed against a person's hair. The balloon is then placed next to a wall. You observe that the balloon sticks to the wall. How is the cause of this phenomenon?
Answer: The balloon attained electrons from the person's hair, and when put near the wall, it polarizes the wall so that electrons in the wall move farther away. Then electrons in the balloon are attracted to positively charged surface of the wall.
Difficult
Question: Draw polarization of a plastic block when a negatively charged balloon is placed near it.
Answer: Since a plastic block is an insulator, electrons in the block move small distance from their original location unlike conductors, in which electrons move freely and move to a surface when polarized.
Connectedness
Polarization is one of the main ideas in electricity and occurs whenever there is a charged object near another object. Real life applications includes when a balloon sticks to a wall after it is charged. Balloon causes the wall to polarized, and it is attracted to it in the air.
Polarization is connected to my major, mechanical engineering, since using charged object as a material for designing something would require the person to take consideration possible interaction or attraction of the invention with other objects due to polarization.
Polarization is a process that is used for certain charging methods. For example, induction charging is when polarization causes a neutral object to be a induced dipole, and the positive part of it attracts electrons, making the object to be negatively charged.
History
Benjamin Franklin was the first person to use "positive" and "negative" in describing charges and came up with principle of conservation of charges. In 1897, J.J. Thomson experimented with cathode rays and found out that electrons exist in the rays. In 1911, Ernest Rutherford discovered that atoms have a concentrated positive center with protons fixed inside nucleus.
See Also
Further Reading
Physics of Dielectrics for the Engineer by Roland Coelho
Application
http://www.nrcresearchpress.com/doi/abs/10.1139/t89-067?journalCode=cgj#.VmFE97mFPIU
External Links
https://courses.cit.cornell.edu/ece303/Lectures/lecture7.pdf
Reference
http://www.physicsclassroom.com/class/estatics/Lesson-1/Polarization
https://www.youtube.com/watch?v=3xSIA5UVAo8
http://scienceline.ucsb.edu/getkey.php?key=408
https://www.aip.org/history/gap/Franklin/Franklin.html
Matter and Interactions Fourth Edition by Ruth W. Chabay, Bruce A. Sherwood