Scattering: Collisions in 2D and 3D: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
Experiments that involve scattering are often used to study the structure and behavior of atoms, nuclei, as well as of other small particles. In an experiment like such, a beam of particles collides with other particles. If it is an atomic or nuclear collision, we are unable to observe the curving trajectories inside the tiny region of interaction. Instead, we can only truly observe the trajectories before and after the collision. This is only possible because the particles are at a farther distance apart and have a very weak mutual interaction; this essentially means that the particles are moving almost in a straight line. A good example which demonstrates scattering is the collision between an alpha particle (the nucleus of a helium atom) and the nucleus of a gold atom. One will understand this phenomenon more in depth after first understanding the Rutherford Experiment which will get touched on later. | Experiments that involve scattering are often used to study the structure and behavior of atoms, nuclei, as well as of other small particles. In an experiment like such, a beam of particles collides with other particles. If it is an atomic or nuclear collision, we are unable to observe the curving trajectories inside the tiny region of interaction. Instead, we can only truly observe the trajectories before and after the collision. This is only possible because the particles are at a farther distance apart and have a very weak mutual interaction; this essentially means that the particles are moving almost in a straight line. A good example which demonstrates scattering is the collision between an alpha particle (the nucleus of a helium atom) and the nucleus of a gold atom. One will understand this phenomenon more in depth after first understanding the Rutherford Experiment which will get touched on later. | ||
== | ==Impact Parameters== | ||
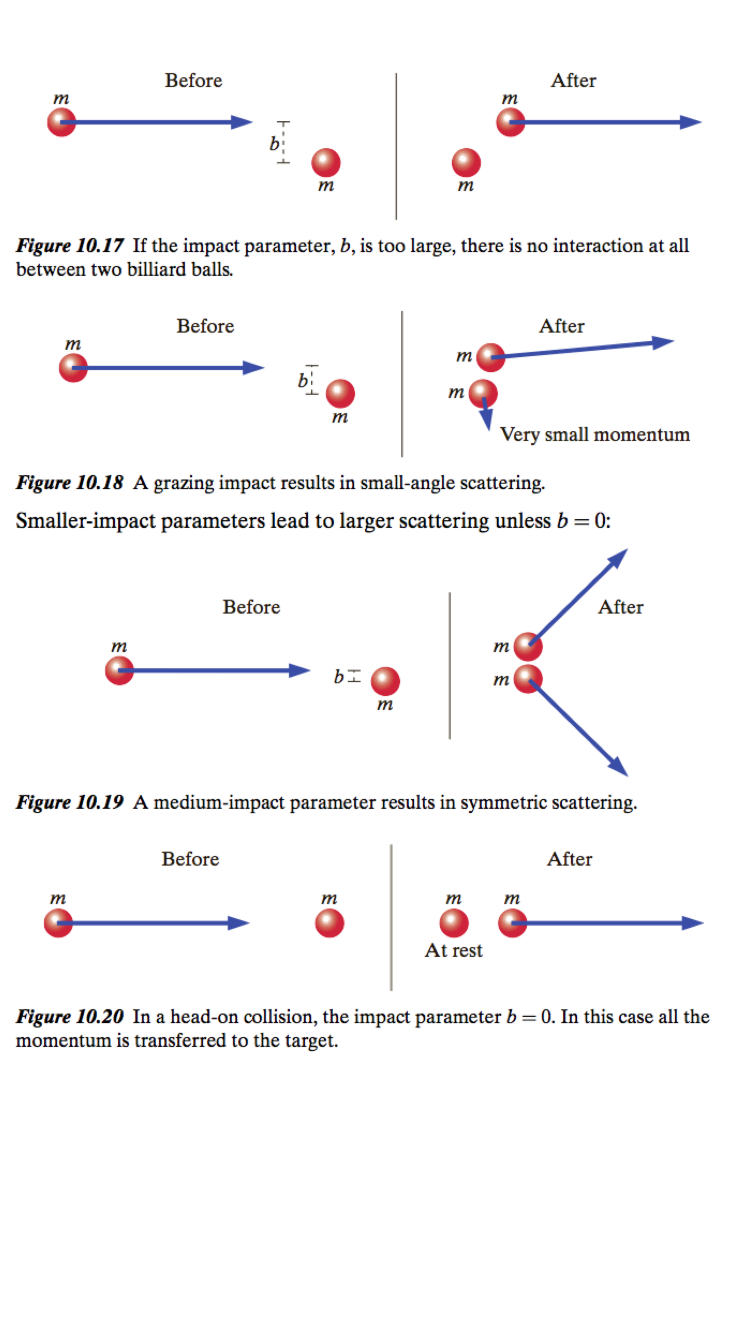
"The distance between centers perpendicular to the incoming velocity is called the “impact parameter” and is often denoted by b. A head-on collision has an impact parameter of zero. Here in Figures 10.17–10.20 are possible elastic collisions between two billiard balls, for various impact parameters:" | |||
"The smaller the impact parameter, the more severe is the collision, and the larger the deflection angle of the incoming particle (larger “scattering”), except for a head-on collision, where if the masses are equal the incoming ball stops dead and the target ball gets the entire momentum." | |||
The collision of an alpha particle (helium nucleus) with the nucleus of a gold atom | The collision of an alpha particle (helium nucleus) with the nucleus of a gold atom | ||
Revision as of 21:50, 5 December 2015
Claimed by: Andreas Ward
The Main Idea
Unlike normal collisions, atomic and nuclear collisions are far too small to observe the curving trajectories of the interacting particles. The only thing that can be noticed is the initial and final states of the interaction. Scattering experiments are incorporated in the world of collisions to be able to study the minute details (structure) of atoms, nuclei, and other tiny particles as the interact with one another.
Experiments that involve scattering are often used to study the structure and behavior of atoms, nuclei, as well as of other small particles. In an experiment like such, a beam of particles collides with other particles. If it is an atomic or nuclear collision, we are unable to observe the curving trajectories inside the tiny region of interaction. Instead, we can only truly observe the trajectories before and after the collision. This is only possible because the particles are at a farther distance apart and have a very weak mutual interaction; this essentially means that the particles are moving almost in a straight line. A good example which demonstrates scattering is the collision between an alpha particle (the nucleus of a helium atom) and the nucleus of a gold atom. One will understand this phenomenon more in depth after first understanding the Rutherford Experiment which will get touched on later.
Impact Parameters
"The distance between centers perpendicular to the incoming velocity is called the “impact parameter” and is often denoted by b. A head-on collision has an impact parameter of zero. Here in Figures 10.17–10.20 are possible elastic collisions between two billiard balls, for various impact parameters:" "The smaller the impact parameter, the more severe is the collision, and the larger the deflection angle of the incoming particle (larger “scattering”), except for a head-on collision, where if the masses are equal the incoming ball stops dead and the target ball gets the entire momentum."
The collision of an alpha particle (helium nucleus) with the nucleus of a gold atom
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
[[Category:Collisions] (Main page)
Further reading
Matter and Interactions, Volume I: Modern Mechanics, 4th Edition. (Chapter 10.6)
External links
References
Chabay, Ruth W., Bruce Sherwood. Matter and Interactions, Volume I: Modern Mechanics, 4th Edition. Wiley, 19/2014.