Systems of Charged Objects
This Page belongs to Laura Breithaupt
In many cases in the real world, there are more than one charge contributing to an interaction. However, in some simple cases, one can choose a system (and ignore external forces) by choosing a system of charges and observing the changes of Kinetic energy (K_sys) and Potential energy (U).
The Main Idea
In physics, a physical system is a region of the physical universe to be analyzed, and everything outside the system is known as the "surroundings." The same logic can be applied to a system of charges; only energy changes within the system need to be analyzed.
A Mathematical Model
ΔK_sys + ΔU = 0 (zero work done by surroundings)
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Example 1
Consider a system of two uniformly charged plates of a capacitor and a single proton. In the region between the plates, there is nearly uniform electric field and external forces can be ignored. The proton is traveling through the right between the plates.
While the proton is between the plates, an electric force is acting on it. The direction of the force is to the right (Electric field points to the right: away from the positive plate and toward the negative plate) F_electric = Q_proton * E = e * E
Question: Will the Kinetic Energy (K) of the proton increase or decrease as a result of this force?
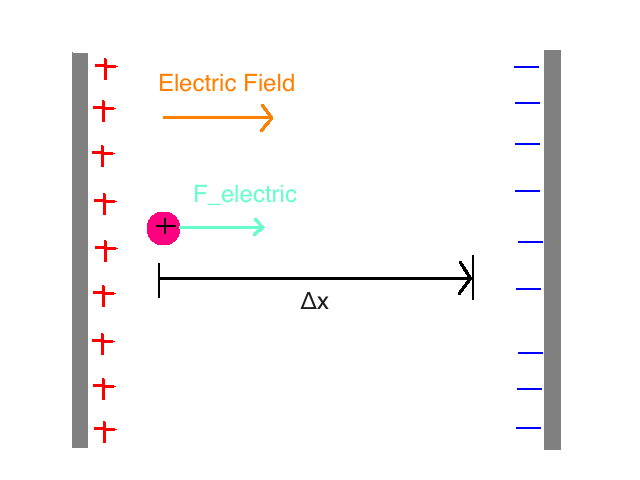
Answer: Because the force is in the same direction as the motion of the particle (the displacement, Δx), the Kinetic Energy will increase.
ΔK_proton + ΔU_electric = 0
The change in electric potential energy is equal to the negative of internal work of the system.
ΔU_electric = -(W_internal) = -(F_electric * Δx) ΔU_electric = -(e * E * Δx)
Because E and Δx are in the same direction, their product is positive.
ΔK_proton + ΔU_electric = 0 ΔK_proton = -(ΔU_electric) ΔK_proton = e * E * Δx , which is a positive value
Kinetic energy of the proton increases because ΔK_proton is positive.
Example 2
Consider a system of two uniformly charged plates of a capacitor and a single electron. In the region between the plates, there is nearly uniform electric field and external forces can be ignored. The electron is traveling through the right between the plates.
While the electron is between the plates, an electric force is acting on it. The direction of the force is to the left (Electric field points to the right: away from the positive plate and toward the negative plate) F_electric = Q_electron * E = -(e * E)
Question: Will the Kinetic Energy (K) of the electron increase or decrease as a result of this force?
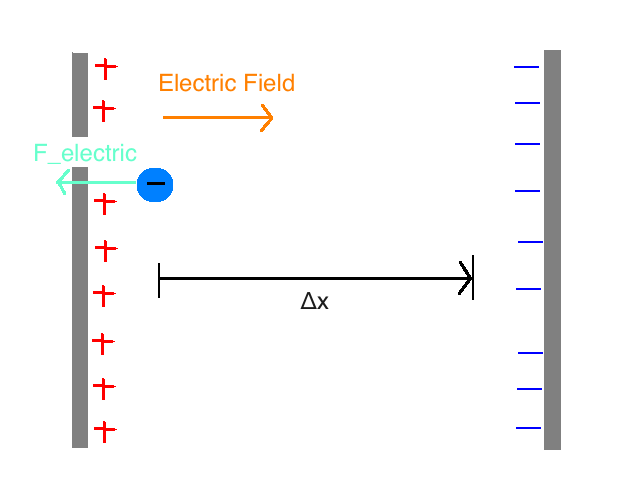
Answer: Because the force is in the opposite direction as the motion of the particle (the displacement, Δx), the Kinetic Energy will decrease.
ΔK_electron + ΔU_electric = 0
The change in electric potential energy is equal to the negative of internal work of the system.
ΔU_electric = -(W_internal) = -(F_electric * Δx) ΔU_electric = -(-(e * E) * Δx) = e * E * Δx
Because E and Δx are in opposite directions, their product is negative.
ΔK_electron + ΔU_electric = 0 ΔK_electron = -(ΔU_electric) ΔK_electron = -(e * E * Δx) , which is a negative value
Kinetic energy of the electron decreases because ΔK_electron is negative.
Connectedness
The law of conservation of energy and the utility of a system of particles is applicable in many situations outside of a strictly "physics" scenario. In my biochemistry classes, we study the interactions of protons and electrons. While we do not focus on "electric fields" per se, the energy of bonds and electrical potential energy are still used to describe the interactions we study. Chemical potential energy has to do with the position of a charge relative to other charges. In a chemical bond, the charges are separated at a distance where there is equilibrium between the electrostatic attractions and repulsions. If one of the particles is moved from this "resting" equilibrium position, it gains electrical potential energy.
History
Leibniz first defined the concept of "energy" as a "vis viva" or living force. He stated that it was mass times its velocity squared.
In 1829, Coriolis modernized the notion of "kinetic energy" as we know it today. In 1853, Rankine developed the idea of "potential energy" of a system.
Using energy as a method of relating masses, displacement, velocity, and other vector quantities as scalars simplifies calculations. Thinking of physical interactions as changes of energy within a system (and also the external forces as 'work' on a system) revolutionized the way many problems were approached and solved.
See also
Further reading
Eugene Hecht, “An Historico-Critical Account of Potential Energy: Is PE Really Real?” The Physics Teacher 41 (Nov 2003): 486-93.
Katalin Martinás, “Aristotelian Thermodynamics,” Thermodynamics: history and philosophy: facts, trends, debates (Veszprém, Hungary 23-28 July 1990), 285-303.
References
Chabay, Ruth and Bruce Sherwood. Matter and Interactions Volume II: Electric and Magnetic Interactions. 4th ed. Danvers, MA: John Wiley and Sons, Inc., 2015. Print.