Photon Emission
Spontaneous Photon Emission
Page in progress by kylerasmussen44
Revised by Sunny Chen (schen474) 2017
Photon Emission is a process that occurs when an atom or other quantum system goes down an energy level, and releases a photon. This process is often incited by the absorption of a particle whose energy causes an atom to increase its energy level; in this case, spontaneous photon emission would move the atom to a lower energy level, closer to its initial state (i.e., ground state). Photon emission is responsible for most of the light that we see, being given numerous names such as luminescence, fluorescence, and phosphorescence.
The Main Idea
Spontaneous photon emission is fundamentally a quantum process, with its principles first being discovered by Paul Dirac. This phenomenon can best be described by using the theory of zero-point energy, or ground state energy. As an electron or similar particle gains enough energy to move out to a higher energy orbit then back to its ground state, it has to lose energy to fall back down into the lower orbitals. The only way it can do this is by releasing a photon. As the particle experiences an electronic transition from the excited state to the ground state, energy is released in the form a photon.
A Mathematical Model
For a simple mathematical model, a light source is in an excited, higher energy state with energy [math]\displaystyle{ E_2 }[/math], and it decays into a lower energy level with energy [math]\displaystyle{ E_1 }[/math]. This change in energy is expressed in the form of an emitted photon with the energy being calculated as the angular frequency [math]\displaystyle{ \omega }[/math] times the reduced Planck constant [math]\displaystyle{ \hbar = {{h}\over{2\pi}} = 1.054\ 571\ 800(13)\times 10^{-34}\text{ J⋅s} = 6.582\ 119\ 514(40)\times 10^{-16}\text{ eV⋅s} . }[/math]
Examples
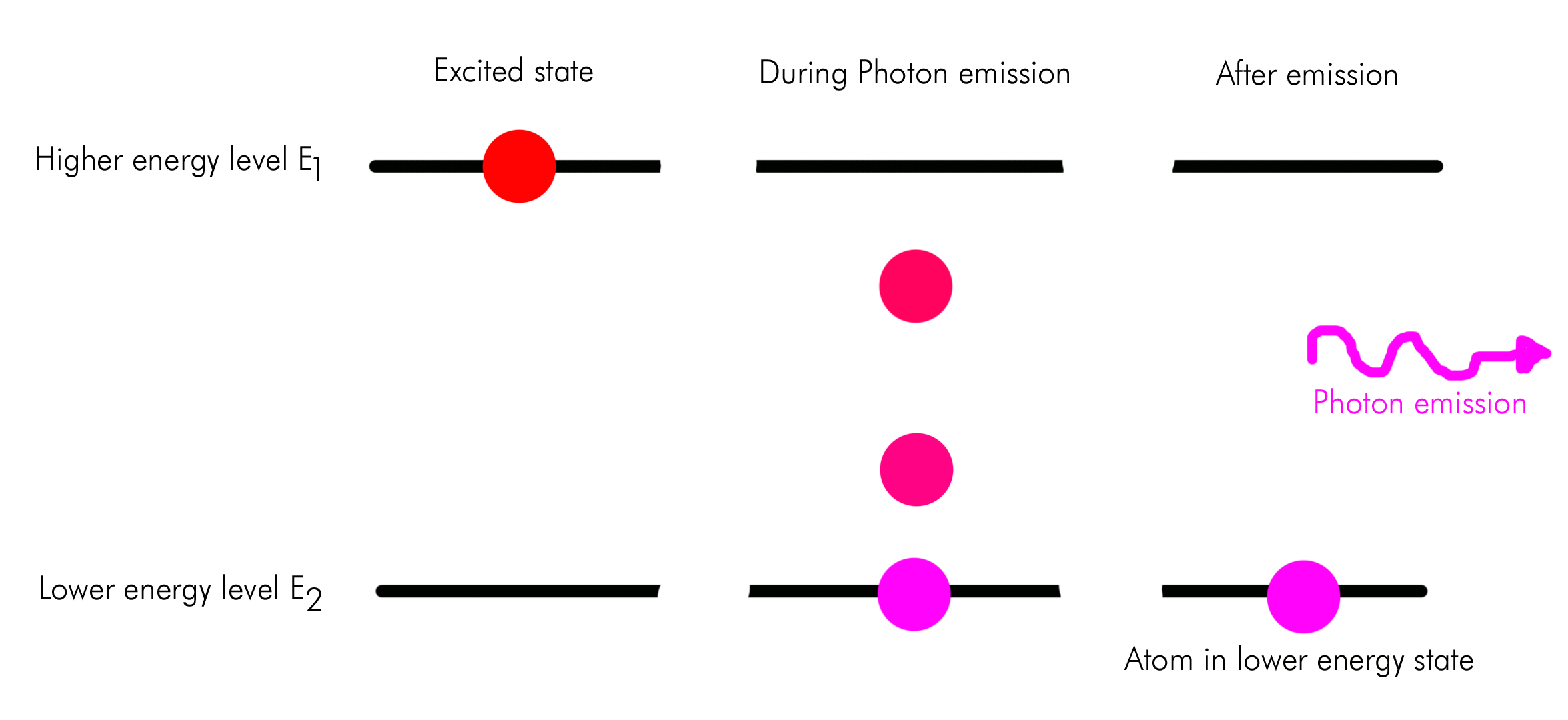
A visual example of spontaneous photon emission is shown below.
Connectedness
How is this topic connected to something that you are interested in?
This topic is interesting because it is how most of the light that we see is emitted. This is the basis of our vision and our world. Without this principle, we would not understand how electromagnetic radiation affects our bodies.
How is it connected to your major?
This is not connected to my major of Industrial Engineering, but I found this topic extremely interesting, and I wanted to explore the principle.
Is there an interesting industrial application?
There are many industrial applications which connect photon emission. This principle is used in everything that emits electromagnetic radiation, including lasers, glow in the dark paint, and anything that works by exciting particles in order to produce light.
History
Although photon emission has always existed, we have never understood how it works. This is one topic that we can safely say that we discovered, not invented. The principle was first explained by Paul Dirac in his quantum theory of radiation. In 1963, the Janes-Cummings model was developed. This model describes the system of a two-level atom interacting inside a quantized optical cavity. This theory is exclusive in the fact that in earlier studies of the quantum theory of radiation, only the atom was quantized, and the field was treated as a definite function of time. This change had a profound effect on quantum electrodynamics, allowing the studies of cavity quantum electrodynamics.
Further Reading and External links
University of Oregon interactive demonstration of photon emission and atomic energy levels.
References
- Jaynes, E. T.; Cummings, F. W. (1963). "Comparison of quantum and semiclassical radiation theories with application to the beam maser". Proceedings of the IEEE. 51 (1). doi:[1]
- Milonni, Peter W. (1984). "Why spontaneous emission?" (PDF). Am. J. Phys. 52 (4): 340. Bibcode:1984AmJPh..52..340M. doi:[2]
- 3. F. van Driel, G. Allan, C. Delerue, P. Lodahl,W. L. Vos and D. Vanmaekelbergh, Frequency-dependent spontaneous emission rate from CdSe and CdTe nanocrystals: Influence of dark states, Physical Review Letters, 95, 236804 (2005). doi:[3]