Josiah Willard Gibbs
Josiah Willard Gibbs was an American born scientist known primarily for his studies in thermodynamics and development of statistical mechanics. His findings paved the way for future discoveries in quantum mechanics and theoretical physics.

Personal Life
Early Life
Josiah Willard Gibbs was born to Josiah Gibbs Sr. and Mary Anna Van Cleve on February 11, 1839 in New Haven, Connecticut. As a child he lived a relatively privileged life with his four older sisters. Education was encouraged in his family as not only was his father a professor at Yale, but also had one relative who held a position as president of Harvard and another relative who was the first president of Princeton.
His classmates at The Hopkins School, a small private school in New Haven, often described him as quiet and "intellectually absorbed." His fragile pulmonary and overall health likely contributed to his introverted demeanor as it prevented him from interacting with his peers.
Later Life
Gibbs continued his pursuit of education when he matriculated to Yale when he was only 15 years old (in 1854). There, he began to pursue engineering research while also receiving numerous awards for his exceptional academic performance in Latin and Mathematics. After successfully completing his undergraduate career, Gibbs continued with graduate studies at Yale. In 1863, at the age of 19, Josiah Willard Gibbs became the first American to receive a doctorate in engineering. It is evident that his academic accomplishments were made possible through his intellect and work ethic, but it is worth noting that his health problems prevented him from serving in the Civil War and allowed him to focus on his studies.

Several years after finishing his graduate work and completing three years as a tutor at Yale, Josiah Willard Gibbs journeyed to Europe with his sisters where he attended lectures on mathematics and physics. After his three years abroad, Gibbs returned to America with a more european view of science. This was one of the reasons that despite his future scientific work, notoriety came very slowly, if at all, in the United States.
Gibbs became a pioneer in another aspect of academia when in 1871, he became the first professor of Mathematical Physics in the United States. Due to his financial security from his inheritance from his parents, he taught without pay for nine years at Yale.

During his remaining years, Josiah Willard Gibbs maintained his reclusive lifestyle. Not only did he never marry, but he also remained living in his childhood home with his older sister and her husband. Despite a few vacations to the Adirondacks and New Hampshire, Gibbs spent the rest of his life in New Haven either working at Yale or in his home. He died April 28th 1903 from acute intestinal obstruction.
Scientific Work
Early Scientific Career
Despite his intellect, Josiah Willard Gibbs did not attract much attention in the scientific community for several reasons. One of these being that American colleges at the time did not encourage research, another being academia focused more on "practical" questions rather than theoretical ones. Additionally, his writings were esoteric in a unique sense; chemists found his papers to be to mathematical, while mathematicians found them to be too scientific.
However, due to his theoretical capabilities, he was able to develop one of his most prominent achievements. By analyzing James Watt's steam engine governor and analyzing its equilibrium, he bagan to develop an equation to calculate and quantify the equilibriums of chemical processes. (This will be discussed in further detail shortly).
Scientific Contributions
As mentioned before, his brilliance in the scientific field was unfortunately not appreciated at the time however made lasting impacts on the scientific community.
Arguably, his most famous contribution was the concept of Gibbs Free Energy. In short this relates a systems energy and entropy. He believed that understanding a systems equilibrium (the maximum of a systems' entropy) was essential in understanding the system as a whole. His equations developed allow one the calculate a system's free energy, how fast the reaction will occur, its direction, and spontaneity. He was the first person to derive a differential equation relating temperature, entropy, energy, pressure and volume which can be simplified into the following equation:
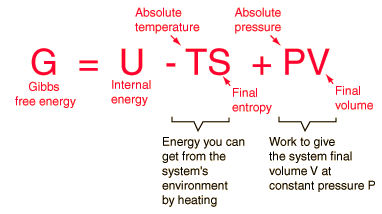
This theory can also be used to find the change in Gibbs Free Energy as shown in the following equation:
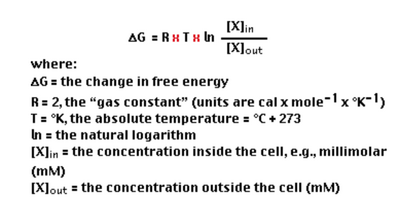
If the numerical value for Delta G is negative, the reaction is spontaneous. If the value is positive, the reaction is non-spontaneous. If the value is zero, the system is in its equilibrium state.
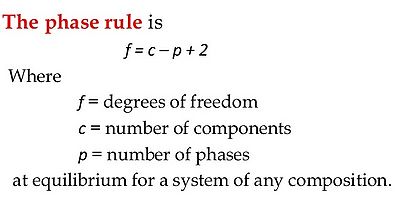
Another famous theory he developed is known as Gibbs Phase Rule. This theory applies to heterogenous systems in thermodynamic equilibrium and succinctly calculates the number of possible phases in a system.
In his goal to find simpler approaches to science, Gibbs played a pivotal role in popularizing the use of vector notation in physics. This more straight forward method replaced the more complex method of quaternions which were previously used.
Significance of Gibbs' Scientific Contributions
Gibbs' contributions forever changed the way the world looks at and studies science and laid the groundwork for many future scientists' discoveries/theories. His work was a main factor in the development of physical chemistry as a field of study. Although Gibbs contributed new ideas to academia, he also aimed to simplify previous theories and topics, such as quaternions.
It is quite noteworthy that Albert Einstein, one of the most famous modern scientists, called Gibbs, "the greatest mind in American history."
Recognition
Notable papers
Josiah Willard Gibbs could be considered a late bloomer in the scientific community. Although his papers became quite influential, his first paper was not published until he was 34 years old. The following are a few of his most famous papers:
1873: Graphical Methods in the Thermodynamics of Fluids (his first major paper)
1873: A Method of Geometrical Representation of Thermodynamic Properties of Substances by Means of Surfaces
1876: On the Equilibrium of Heterogeneous Substances (undoubtedly Gibbs' most famous paper)
1902: The Elementary Principles of Statistical Mechanics (this laid the foundation for new branch of theoretical physics, statistical mechanics, and paved way for developments in quantum mechanics and Maxwell’s theories)
Awards
1858: Connecticut Academy of Arts and Sciences
1892: London Mathematical Society Honorary Member
1897: Fellow of Royal Society
1901: Royal Society of Copley Medal (This is the highest award from Royal Society of London awarded for either most important scientific discovery or greatest contribution by experiment)
Connectedness
Josiah Willard Gibbs contributions to the scientific community are so much more influential than the average person could ever imagine. As mentioned previously, his findings allowed future scientists, such as Einstein, to make their breakthrough discoveries. His theories impact daily life on the micro and macroscopic scale. With regards to industrial applications, his findings are crucial as they accurately predict the outcomes of reactions.
As a Materials Science and Engineering major, much of what Gibbs studied and discovered is interconnected with my studies. For example, Gibbs phase rule is ever-present in phase diagrams of materials. I find it admirable that he not only persevered, but also remained humble throughout his life despite his lack of recognition.
See also
Further reading
Josiah Willard Gibbs: The History of a Great Mind by Lynde Phelps Wheeler
Elementary Principles in Statistical Mechanics by Josiah Willard Gibbs
Scientific Papers of J. Willard Gibbs by Josiah Willard Gibbs
Memoir of the Gibbs family of Warwickshire, England, and United States of America by Josiah Willard Gibbs