Effects of Radiation on Matter
Radiations and their interactions with matter, Venkat Natarajan
The Main Idea
Radiation consists of 2 components, electric fields and magnetic fields. The question being answered is "How do these radiations interact with matter?" We know that an atom is basically a nucleus and its electron cloud, but the nucleus is so small that the chances of radiations striking the nucleus is negligible. On the other hand, the peripheral electrons stand a much greater chance (about [math]\displaystyle{ 10^9 }[/math]) of getting impacted by radiation. The denser the material, the higher number of subatomic particles which results in a greater probability of interaction.
Description of The Interaction
Energy can neither be created nor destroyed, so when a photon encounters a particles, it transfers its energy to the particle. This energy can be returned to scattered in a different direction, transmitted through, or absorbed through the material. The force that these particles experience is caused by the electric field, which we have studied before : [math]\displaystyle{ F=qE }[/math]
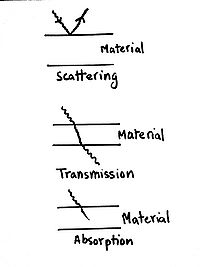
Scattering
Electromagnetic radiations can be reflected or scattered by the material. The laws that govern this are the same as those that govern reflection of light, as explained in Optics.
Transmission
This happens when the radiation is allowed to pass through. Depending on the material and type of radiation, different amounts of radiation are transmitted. If the material does not absorb any, it is said to be transparent. However, perfect transparency is only theoretical as some energy (which may be unnoticeable) is absorbed or reflected.
Absorption
Here, the material absorbs the energy, totally or partially. This usually results in some sort of change in the material.
Note: If the energy is below a certain level, no physical interaction is possible. This level is the threshold energy.
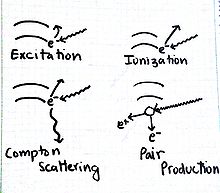
Results of Electromagnetic Radiation
Excitation
This occurs when an electron absorbs just the right amount of energy "jumps" to a higher energy level.
Ionization
This process happens when an electron absorbs an amount of energy that is higher than the ionization potential, resulting in the emission of an electron. Consequently, the ion formed may be highly reactive.
Compton Scattering
The effect is the emission of an electron and a photon of lower energy.
Pair Production
A phenomenon that follows the energy-mass equivalence [math]\displaystyle{ E=mc^2 }[/math]. A photon with really high energy results in the formation of particles, an electron and a positron. Note, the net charge of the products is zero, just as the charge of a photon is zero.
Energy of a Photon
Each photon carries the same amount of energy, hence the term "quantized". [math]\displaystyle{ E=hf }[/math] where h= Planck's constant [math]\displaystyle{ = 6.626*10^-34 J }[/math] and [math]\displaystyle{ f= frequency }[/math]
Importance of The Frequency of Electromagnetic Radiation
Depending on the frequency, its effect on matter varies. For example-
- Waves with a high frequency of around 10^15 Hz interact with organic molecules of your eye, allowing you to see light. However, the same waves have no effect on satellites.
- Waves with a frequency of around 10^6 Hz help in communication and are called radio waves. Antennas are able to pick them up and allow us to listen to music that is played from radio stations. As we have observed through experience, radio waves have no effect on our eyes.
- X-rays have an extremely high frequency and pass through most of our body, except for the highly dense areas, like our bones. This allows us to "look through" people.
All of these phenomena are explained by resonance, which basically tells us that if the interacting matter/surface receives radiation at the same frequency as its natural frequency thereby strengthening its vibrational motion.
Connectedness
I am a biomedical engineer and the medical devices that are used to detect tumors, fractures etc rely on the way different electromagnetic waves behave when they strike the human body. It allows us to use non-invasive methods instead of having to get into surgery.
History
Electromagnetic radiation occurs in multiple forms, and each type was discovered by a different person.
- Wilhelm Roentgen is credited with discovering and naming X-rays.
- Johann Wilhelm Rittler discovered Ultraviolet rays using a prism and William Herschel discovered infrared waves in a similar fashion.
See also
Further reading
External links
http://www.avogadro.co.uk/light/matter/quantised.htm
References
Matter & Interactions, Vol. II: Electric and Magnetic Interactions, 4nd Edition by R. Chabay & B.Sherwood (John Wiley & Sons 2015) http://resources.yesican-science.ca/trek/radiation/final/index_em_matter.html https://www.nde-ed.org/EducationResources/CommunityCollege/RadiationSafety/theory/interaction.htm