Rutherford Experiment and Atomic Collisions
Claimed by: dveludhandi6 (Fall 2017)
Rutherford's Gold Foil Experiment
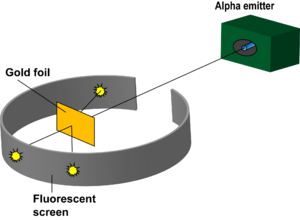
Rutherford's Gold Foil Experiment helped detect that there was a large positively charged mass in the center of an atom: the nucleus. The experiment was done through the use of atomic collisions. Under the instruction of Rutherford, Hans Geiger and Ernest Marsden pointed a beam of alpha particles at a thin foil of metal and measured the scattering pattern by using a fluorescent screen. The scientists noted that some alpha particles bounced in random directions. This was not originally hypothesized due to the idea that, at most the alpha particle should experience only a 90° scattering angle. This helped lead to the discovery of the nucleus and a highly compact positively charged center.
History
Around the early 1900s, very little was known about atoms besides the ground breaking experiments conducted by J.J. Thompson in 1897. Thompson discovered what we call the electron. He hypothesized that electrons were negatively charged particles. It was also speculated that there must be a positive charge to balance out the negative charge from the electron. This "Plum Pudding Model" was invented by Thompson. This model assumed that matter consists of atoms which are overall positively charged, but with some type of negative electron charge throughout it. The electrons function as the "plum" which was evenly distributed through a positively charged "pudding".
Rutherford studied the particles that uranium and its derivatives emitted and how these particles affected certain materials. Rutherford created a method to record the position of each alpha particle by circling the bombarded object with a ZnS coated sheet. This sheet would emit a flash of light when hit by an alpha particle, allowing Rutherford to accurately measure the deflection of each alpha particle. This gave Rutherford a counting mechanism for theses particles he wanted to study. Rutherford then began to study the angles that negatively charged particles deflected when they collided with a thin metal foil. This was the beginning of his most famous study: the gold foil experiment. Knowing the relative mass of these negatively charged particles and their quick speed, he hypothesized that they would pierce the metal foil but then collide with the atoms dispersed inside the foil resulting in the small deflections. These deflections were extremely small, usually by a degree. In 1911, Ernest Rutherford took this experiment further and worked with his assistants, Hans Geiger and Ernest Marsden to carry out an experiment that tested the plum pudding model. They shot alpha (helium 2+) particles at gold foil in order to measure the deflection of the particles as they come off of the other side. They decided to see if these deflections could occur at larger angles greater than 90 degrees. Through countless trials, they found an extremely small portion of these deflections to occur at angles larger than 90 degrees. Rutherford wondered how these large deflections occurred and concluded that there existed an extremely small and positively charged area in the atom that resulted in these huge deflections. He eventually named this area the nucleus. What happened during these deflections was that most particles would become slightly deflected by small angles due to the positive atoms. However, some would collide directly with nucleus resulting int the deflections that were greater than 90 degrees. These occurred rarely because the nucleus was such a small size so the probability of these atoms hitting the nucleus was very low. This experiment helped indicate that the atom is made predominantly of empty space with a small nucleus with protons and electrons placed extremely far away from the nucleus in their own cloud. Rutherford devised the name “proton” to describe the positive particles in the nucleus. He thought that a neutral particle existed in the nucleus too, but its existence wasn’t confirmed until 1932 when James Chadwick proved it.
A Mathematical Model
Rutherford modeled the effect the alpha particle has on the electrons of the gold atom. He did this by calculating the potential electric energy between the particle and the atom using the formula below. Rutherford came up with several equations to numerically describe these deflections. Based on the equations below, the number of particles scattered at a certain angle is directly proportional to the thickness of the metal foil and the square of the nucleus’ charge but inversely proportional to the particle’s velocity raised to the fourth power.
[math]\displaystyle{ {U_{elec}} = {\frac{1}{4πε_0}}{\frac{q_{α}q_{Au}}{r}} }[/math]
Where:
r = center to center distance between particle and atom
[math]\displaystyle{ {\frac{1}{4πε_0}} = {9*10^9}{\frac {N*m^2}{C^2}} }[/math]
[math]\displaystyle{ {q_α} }[/math] = charge of alpha particle
[math]\displaystyle{ {q_{Au}} }[/math] = charge of gold nucleus
In this instance the charge of the alpha particle is equal to 2e and the charge of the gold particle is equal to 79e.
Another important part of atomic collisions is that they are inelastic collisions. This is shown by the conservation of both momentum and kinetic energy. Take the alpha particle and gold particle for example.
[math]\displaystyle{ {\vec{p_{α,i}}} = {\vec{p_{α,f}}}+ {\vec{p_{Au,f}}} }[/math]
[math]\displaystyle{ {\vec{K_{α,i}}} = {\vec{K_{α,f}}}+ {\vec{K_{Au,f}}} }[/math]
Where [math]\displaystyle{ {\vec{p}} }[/math] is momentum and [math]\displaystyle{ {\vec{K}} }[/math] is kinetic energy.
A Computational Model
Much like the mathematical model, the collision can be modeled computationally using the same formulas. In order to view this better, here is an example of one collision using the Rutherford Scattering Model.
Here is a video of a VPython mode of a continuous stream of alpha particles:
- Rutherford Scattering Continuous Model
from __future__ import division from visual import * import random scene.width=1024 scene.height=760 def alpha():
#CONSTANTS
G = 6.7e-11
mEarth = 6e24
mcraft = 9e30
deltat = 10
t = 0
pscale = 1/5
fscale = 500000
dpscale = 100
#OBJECTS AND INITIAL VALUES
rando = random.uniform(-5,5)
box(pos=(-48.4e7,0,0), length=3e8, height=1e8, width=3e7)
ringabazinga = ring(pos=(1,1,1), axis=(0,0,1), radius=3e8, thickness=3e6)
Earth = sphere(pos=vector(0,0,0), radius=6.4e6, color=color.yellow)
scene.range=100*Earth.radius
craft = sphere(pos=vector(-36.4e7,rando*10**7,0), radius=3, color=color.cyan)
vcraft = vector(6e3,0,0)
pcraft = mcraft*vcraft
r = Earth.pos - craft.pos
rmag = mag(r)
rhat = norm(r)
Fmag = G*mEarth*mcraft/rmag**2
Fnet = Fmag * rhat
trail = curve(color=craft.color)
scene.autoscale = 0
# CALCULATIONS
pcraft_i = pcraft + vector(0,0,0)
while t < 1000000:
rate(50000)
deltap = Fnet*deltat
p_init = mag(pcraft)
pcraft+=deltap
p_final = mag(pcraft)
vcraft = pcraft/mcraft
craft.pos += vcraft * deltat
rE = Earth.pos-craft.pos
rmagE=mag(rE)
rhatE=norm(rE)
FmagE = -G*mEarth*mcraft/rmagE**2
Fnet = FmagE * rhatE
Fnet_tangent = ((p_final-p_init)/deltat)*norm(pcraft)
Fnet_perp = Fnet - Fnet_tangent
#crash
if t>100000 and mag(craft.pos) > ringabazinga.radius:
alpha()
trail.append(pos=craft.pos)
t = t+deltat
alpha()
Example Problems
Simple
The scattering of alpha particles from nuclei is mathematically modeled from the Coulomb force and treated as an orbit. For a ZnS detector at a specific angle with respect to the incident beam, the number of particles per unit area striking the detector is given by the Rutherford formula:
[math]\displaystyle{ N(θ) = {\frac{N_inLZ^2k^2e^4}{4r^2KE^2sin^4(θ/2)}}
}[/math]
where
[math]\displaystyle{ N_i = \text {number of incident alpha particles} }[/math]
[math]\displaystyle{ n = \text {atoms per unit volume in target} }[/math]
[math]\displaystyle{ L = \text {thickness of target} }[/math]
[math]\displaystyle{ Z = \text {atomic number of target} }[/math]
[math]\displaystyle{ e = \text {electron charge} }[/math]
[math]\displaystyle{ k = \text {Coulomb's constant} }[/math]
[math]\displaystyle{ r = \text {target to detector distance} }[/math]
[math]\displaystyle{ KE = \text {kinetic energy of alpha} }[/math]
[math]\displaystyle{ θ = \text {scattering angle} }[/math]
Find the number of particles per unit area striking the detector given the following values:
[math]\displaystyle{ N_i = 5 }[/math] alpha particles
[math]\displaystyle{ n = 8.4866 * 10^{22} \text {atoms in 1} cm^3 }[/math]
[math]\displaystyle{ L = 1 cm }[/math]
[math]\displaystyle{ Z = 26 }[/math]
[math]\displaystyle{ e = -1 }[/math]
[math]\displaystyle{ k = 8.988 * 10^9 }[/math]
[math]\displaystyle{ r = 10 cm }[/math]
[math]\displaystyle{ KE = (1/2)*m*v^2 }[/math] where [math]\displaystyle{ v_a = 1.53 * 10^7 m/s \text {and mass of the alpha particle is} 6.64424*10^27 kg }[/math]
[math]\displaystyle{ θ = 0.18 degrees }[/math]
Solution
Plug each number into the equation (make sure units cancel).
[math]\displaystyle{ N(0.18) = {\frac{5*8.4866*10^{22}*1*26^2*{(8.988*10^9)}^2*-1^4}{4*10^2*{((1/2)(6.64424*10^27){(1.53*10^-7)}^2)}^2*sin^4(0.18/2)}} }[/math]
= 1.57341 * 10^27 particles striking the surface per cm
Difficult
A proton and an electron are a distance [math]\displaystyle{ {7.2*10^{-9}m} }[/math] apart. What is the electric potential energy of the system consisting of the proton and the electron?
Solution
[math]\displaystyle{ {U_{elec}} = {\frac{1}{4πε_0}}{\frac{q_{+}q_{-}}{r}} }[/math]
[math]\displaystyle{ {U_{elec}} = {9*10^{9}}{\frac {N*m^2}{C^2}}*{\frac{1.6*10^{-19}*(-1.6*10^{-19})}{7.2*10^{-9}}} = {-3.2*10^{-28}}{J} }[/math]
Connectedness
This topic is related to the study of chemical engineering. Without the discovery of the nucleus, any progress in this field would be limited based on the interaction of atomic particles. This would also hinder the field medicine for very similar reason. Much of the understanding of sciences has its roots in the understanding of the atom and its functions. This experiment and the idea of atomic collisions helped to widen the atomic grasp. One of the best industrial examples of atomic collisions is the Large Hadron Collider.
History
With the knowledge of the plum pudding model of the atom, Ernst Rutherford and a small group of scientists set out to discover the properties behind alpha particles. The experiment, now known as the Gold Foil Experiment, was used to test this in 1911. It involved launching alpha particles at a small piece of gold foil. It was hypothesized that the alpha particle would be deflected at times, but at an angle because it was assumed that the alpha particle was more dense than the gold foil atom. They registered deflected particles through light emissions that would occur when the alpha particle hit the light source. Much to their surprise, some of the alpha particles they launched bounced straight back. This demonstrated that the gold particle was more massive than expected. It led to the discovery that the atom contained a positively charged nucleus. This was a major break through in the study of the atom in that it showed what the atoms composition was and how it act around other atoms.
References
Chabay, R.W., & Sherwood, B.A. (2015). Collisions. In Fiorillo, J. Editor & Rentrop, A. Editor (Eds.), Matter and Interactions (383-410). John Wiley & Sons, Inc.
“Ernest Rutherford.” New Page 2, chemed.chem.purdue.edu/genchem/history/gold.html.
"History of Rutherford Experiment". HyperPhysics. Web. 03 Dec. 2015. Retrieved from: <http://hyperphysics.phy-astr.gsu.edu/hbase/hframe.html>.