Polarization
Written By: tkapadia3 - Tapas Kapadia
Edited By: lwinalski3 - Laura Winalski
Polarization is defined as "the process of separating opposite charges within an object" by the application of an electric field. When the positive and negative charges within an object have become separated, the object is said to be polarized. Due to the physical separation of its positive and negative charges, a polarized object can behave like a dipole, so long as the charge separation continues. Once the electric field causing the polarization is removed and the positive and negative charges are no longer separated, the dipole disappears. For this reason, the process of polarization is said to create induced dipoles. Polarizability is the ease with which the charges in an object can be separated. Polarizability is a constant value, determined experimentally, and is unique to each particular material. The amount of polarization an object experiences, or the dipole moment, is equal to the the polarizability multiplied by the magnitude of the applied electric field. Polarization occurs differently in insulators - materials through which mobile charges cannot flow - and conductors - materials through which mobile charges can flow. It is important to note that the process of polarization in and of itself does not induce charging. Polarization is the redistribution of charges throughout an object; a polarized neutral object is still a neutral object regardless of whether it is an insulator or conductor.
Simple schematic of a polarized molecule, or a dipole
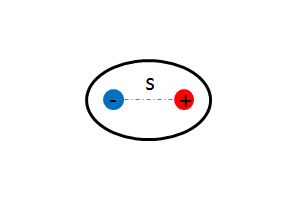
The Main Idea
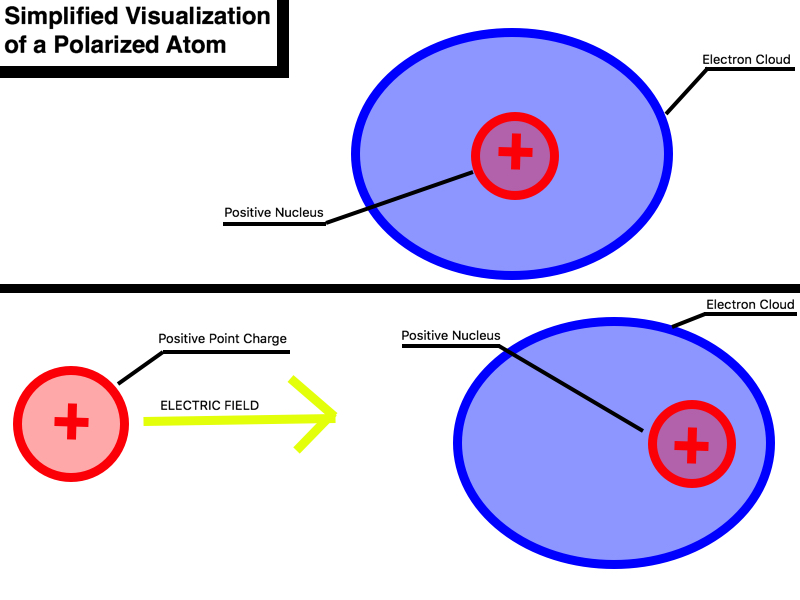
At the atomic level, the application of external charges causes the subatomic particles, namely, positively-charged protons and negatively-charged electrons, to reorient with respect to the applied charge. The external application of a positive charge on the left side of a molecule will result in the electrons moving to the left, as they are attracted to the positive charge, and the protons moving to the right, as they are repelled by the positive charge. Similarly, the external application of a negative charge on the right side of a molecule will result in the protons moving to the right, as they are attracted to the negative charge, and the electrons moving to the left, as they are repelled by the negative charge. (See Charge Interaction)
The externally applied charges are, in truth, electric fields. Positive electric fields point outwards, away from the source, or the object creating the field. Negative charges, on the other hand, create inward pointing electric fields that point towards the source of the field. The external application of a positive electric field to an object will create an outward field, resulting in the movement of the electron closer to the external positive charge and the positively-charged nucleus further away from the external charge. With the charges physically separated in space, an induced dipole forms. When the external positive charge is removed, the induced dipole disappears and the object is once again neutral. This is drastically different from permanent dipoles, in which the positive and negative charges are always physically separated. Polarization and the resulting creation of induced dipoles helps explain the seemingly magical attraction between charged objects and neutral object. A charged object produces an electric field that, when brought near neutral objects, generates induced dipoles. The protons and electrons reorient themselves in the presence of the charged object's electric field and attractive forces can be observed between the two objects.
Polarization in Insulators
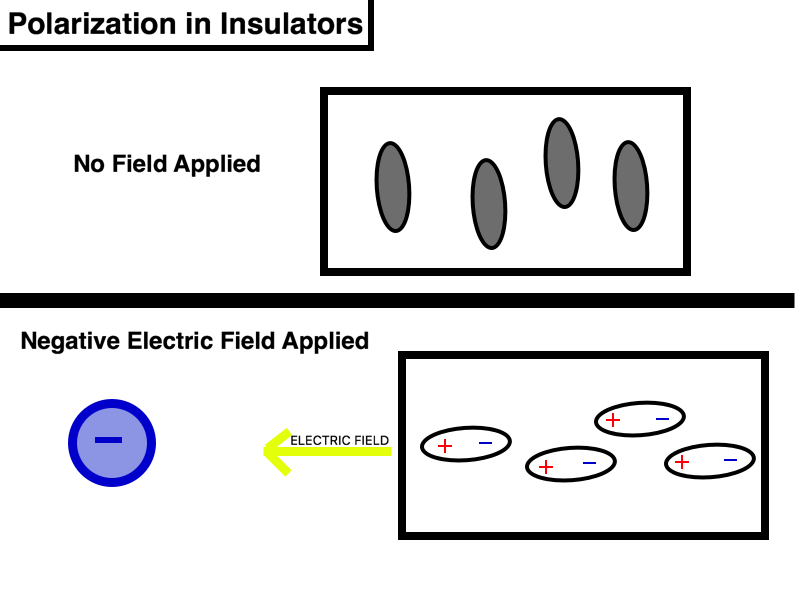
Insulators are materials in which the electrons are tightly bound to the atoms, preventing the movement of charged particles throughout the material. As a result, electricity - or mobile charges - is unable to flow through the object and it is said to "insulate" one object from another. Common examples of insulators include wood, rubber, paper, and glass. Insulators have very low polarizability constants. In the presence of an externally applied electric field, the electrons in an atom shift positions slightly, but are unable to move and become separated from the protons. At most, the electrons can shift one atomic diameter, or 1x10^-10 meters, but they ultimately remain attached to the atoms. This results in the induced polarization of the individual molecules inside the insulator, as the applied electric field has caused the normally neutral object to become polarized. Within each of the individual molecules making up the insulator, dipoles have formed, but the molecules themselves have not moved. The magnitude of the induced polarization is dependent upon the strength of the applied electric field. The stronger the applied electric field, the greater effect it has on the insulator. Although the molecules are not shifting, induced polarization still creates a large effect as there are many molecules to be polarized and therefore, many induced dipoles to form. The separation of the positive and negative charges is proportional to the strength of the external electric field. If the applied electric field is large enough, the induced dipoles will generate their own electric field as illustrated below.
Polarization in Conductors
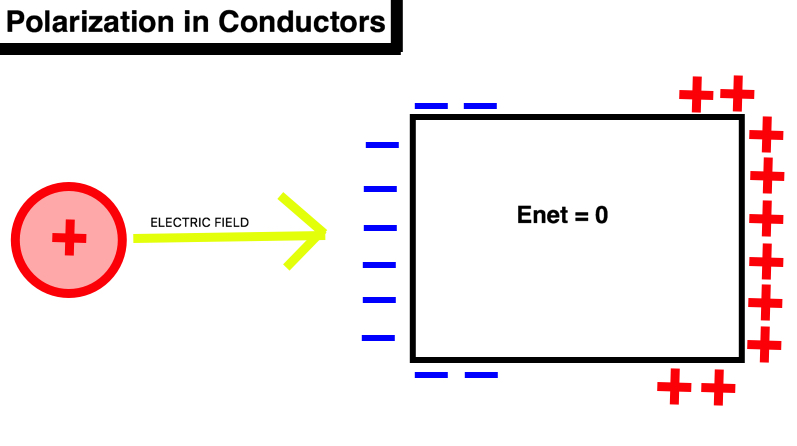
Conductors are materials in which charged particles are able to flow freely, unlike insulators in which charged particles are tightly bound to the atoms. Charged particles within conductors are able to move great distances, and their movement is the basis of electricity. Common examples of conductors include silver, gold, salt water, concrete, and aluminum. As a result of the unrestricted freedom of charged particles, polarization in conductors differs from polarization in insulators. While the electrons in an insulator are capable of reorienting themselves, the electrons in conductors can move great distances and spread across the entire surface in response to the application of external charge. The mobile charges can even accumulate on the outside of the surface of a conductor! The process of polarization may cause the mobile charges to reorient on the surface in such a way that the net electric field goes to zero as the electric field on the surface cancels out the applied electric field. When this happens, the object is said to be in equilibrium and the electrons are no longer capable of moving through the object. The speed with which the mobile charges move due to an applied electric is known formally as drift speed. The drift speed is equal to the the net electric field at the location of the charge multiplied by the mobility of the mobile charges. As made evident by this equation, when the object is in equilibrium, the charges stop moving.
Static Electricity
Static electricity is energy that builds up due to the interaction between charged objects. Simply put, static electricity is an imbalance between positive and negative charges. Because objects are polarizable - whether completely (conductors) or incompletely (insulators), when charged objects come into contact with each other, the electrons and protons within reorient or shift. As illustrated above, opposites attract, meaning negatively charged objects will experience an attractive force towards positively charged objects. On the other hand, similarly charged species will repel each other. When similarly charged objects are brought into contact with one another, the mobile particles will attempt to escape as quickly as possible. This rapid movement of similarly charged particles is known as static shock. Homeowners frequently experience static shock as they walk across a bedroom carpet and build up electrons on their body. When they reach for a doorknob or light switch, they experience static shock as the electrons quickly "escape," or discharge.
A Mathematical Model
Useful formulas for calculating polarization and its effects:
Electric Force: [math]\displaystyle{ \vec{F} = q\vec{E} }[/math] Where "F" is the electric force, "q" is the charge, and "E" is the electric field.
Dipole Moment: [math]\displaystyle{ \vec{P} = \alpha \vec{E} }[/math] Where "P" is the dipole moment, alpha is the polarizability (different for every material), and "E" is the applied electric field.
Drift Speed: [math]\displaystyle{ \vec{v} = \mu E_{net} }[/math] Where "v" is the drift speed, mu is the mobility of the charge, and "Enet" is the magnitude of the net electric field.
Examples
Simple
Determine if the statements below are True or False:
1. Charged particles can flow freely within conductors.
2. The net electric field is equal to 0 when both insulators and conductors are in equilibrium.
3. Excess charges become localized on the surface within insulators, but not within conductors.
4. The average drift speed of a mobile charge is proportional to the magnitude of the net electric field the material.
1. True. There are mobile charges in conductors. Insulators do not have mobile charges as the electrons are bound tightly to the atoms.
2. False. The net electric field is 0 only when conductors are in equilibrium. Insulators are not able to reach equilibrium.
3. False. The excess charges within conductors become localized on the surface. In insulators, the excess charges are anywhere: either on the surface or inside of the material.
4. True. The formula for drift speed - [math]\displaystyle{ \vec{v} = \mu E_{net} }[/math] - includes the mobility of the charge and the magnitude of the net electric field at the location of the mobile charge.
Middling
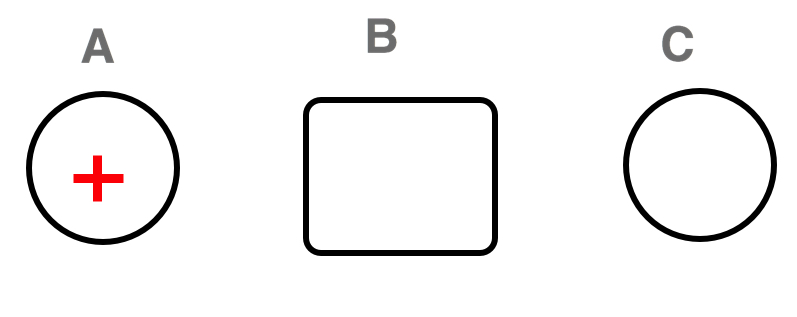
Does a negatively charged rod cause a neutral metal sphere to polarize? If so, show the polarization of the neutral metal sphere, describe the electric field (its magnitude and direction), and electric force caused by the negatively charged rod displayed below.

The electric field points towards the negatively charged rod. The electric force is also pointed towards the charged rod. Thus, the negative mobile charges are pushed to the surface of the far right side of the sphere. The polarization creates a large dipole which has a zero net electric field inside the sphere.
Difficult
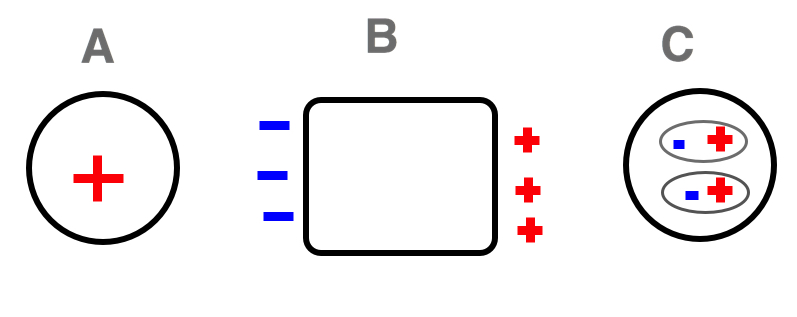
Find and show the polarization of Block B and Sphere C if Sphere A is plastic sphere with a positive charge. Block B is natural metal block while Sphere C is plastic sphere.
The positive charge on Sphere A creates an electric force which drives the positive mobile charges on the block away while attracting negative surface charges on the block. Due to the electric force, the block polarizes in the manner illustrated below: negative charges move to the left, positive charges move to the right. The positive surface charges on the block near sphere C cause induced polarization, forming induced dipoles, as Sphere C is an insulator. If Sphere C were a conductor, the sphere would polarize, but as it stands, a mere reorientation takes places. The negative charges of Sphere C orient close to the positive surface charges of Block B.
Connectedness
How is this topic connected to something that you are interested in?
Polarization helps explain the mystical, yet fascinating, phenomena of attraction between neutral and charged objects. Polarization is evident in our every day lives in many ways. For example, the build up of static charge on your socks as you walk across the carpet and the shock you feel when you touch a door handle as your body discharges. As with the entire field of physics, polarization helps explain he science behind many of the phenomena we experience daily that go unnoticed.
How is it connected to your major?
I am a computer engineering major. Honestly, I am not sure yet what Computer Engineering is all about. However, I am aware the polarization is an important concept in electrical engineering. Polarization of light waves seems to be more connected to my major.
Edit by Laura: As a biochemistry major, it is important for me to understand the interactions between molecules. Whether I am in the analytical laboratory, running mass spectrometry on a sample and need to choose whether to detect negative or positive ions, or I am in the biochemistry laboratory, running gel electrophoresis on a sample of DNA, watching the negative strands move towards the positive electrode and the positive components move towards the negative electrode, the basic principle is the same: CHARGES ARE IMPORTANT. Charges and the interactions between charged molecules are essential for chemistry, for physics, and for, in truth, life as we know it.
Is there an interesting industrial application? The concept of polarization itself has many industrial applications. It is seen in 3D Glasses, Infrared spectroscopy, polarized sunglasses, FM radios, and even laptop screens. There are many, many industrial applications.
Edit by Laura: As stated above, charges and the interactions between charged molecules are essential principles in chemistry. Electrophoresis is the process of separating a mixture using electricity. Charges flow through a sample causing the components to separate based on their charge: positively charged components are attracted to the negative electrode and negatively charged components are attracted to the positive electrode. Gel electrophoresis has played a key role in the development of vaccines and modern medicines. Additionally, it is solely responsible for the separation of DNA: a key tool in forensic identification and genetic testing. Much of modern medicine has come about simply based on the notion of charge and the interaction between positively and negatively charged species.
The basic set up of a gel electrophoresis experiment to separate DNA.
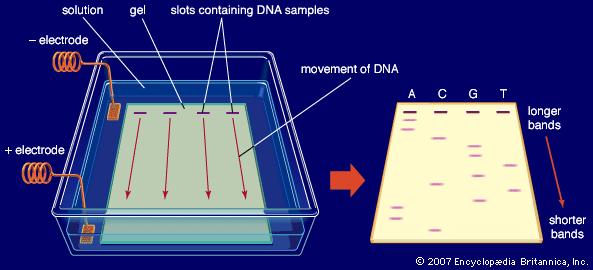
Photo copied from Encyclopedia Britannica.
History
The microscopic process of polarization has expanded into the macroscopic world through light, radio waves, and industry. The macroscopic application of the microscopic phenomenon was first discovered by Etienne Louis Malus, a French physicist in the early 1800s. Malus understood that light is consists of electromagnetic waves, and therefore, contains a range of radiation. The human eye is unable to see all of the waves in the range of light, but Malus used instruments and science to explore light outside of the visible spectrum, the region capable of detection by the human eye. Through his studies, Malus discovered the versatile applications of polarization.
See also
Electric Field. Electric Force. Charge Density. Static Electricity. Gel Electrophoresis.
Further reading
Matter and Interactions Volume II.
External links
References
- Matter and Interactions Volume II.
Unless otherwise stated, images were made by the author or editor.