Internal Energy: Difference between revisions
Mmcintire8 (talk | contribs) (→Simple) |
Mmcintire8 (talk | contribs) |
||
| Line 64: | Line 64: | ||
===Middling=== | ===Middling=== | ||
===Difficult=== | ===Difficult=== | ||
(THIS IS NOT AT ALL PHYSICALLY ACCURATE) | |||
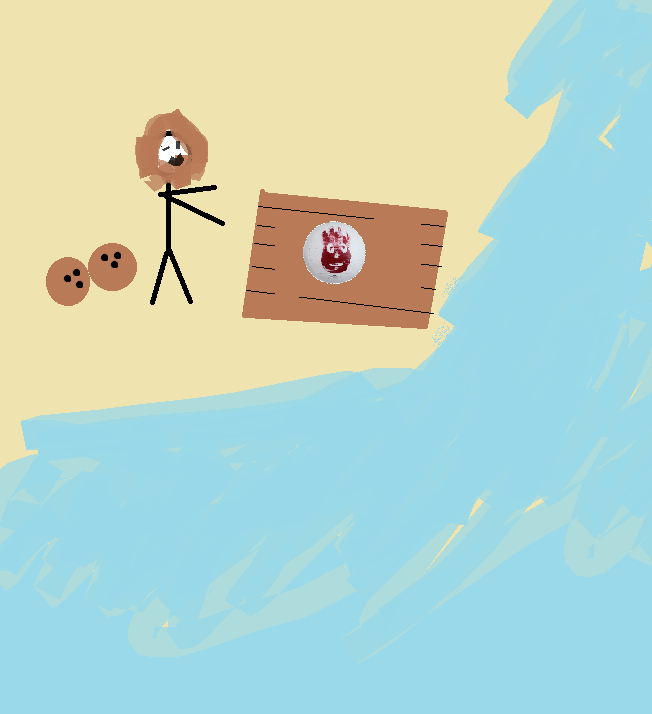
Assume you are stranded on an island. You have built a raft with the last of your energy. You have 2 coconuts that will each give you an estimated 1,500 calories of energy. (A calorie is roughly 4.2*10^3 Joules). You have started moving your 200kg raft across the low-friction sand with inital velocity 2m/s. You have calculated that in order to escape the incoming tide, your raft must be travelling at least 12m/s when it reaches the ocean. You hope to save one coconut for your voyage. Will one coconut give you enough energy to move the craft? | |||
Note:Calories is representative of Chemical Energy, and thus find the minimum change in internal energy required to move the raft to solve this problem. | |||
1)Diagram | |||
[[File:InternalEnergy2.png]] | |||
==Connectedness== | ==Connectedness== | ||
Revision as of 23:21, 5 December 2015
Claimed by Michelle McIntire M06
Main Idea
Internal Energy, as referred to in Intro Physics, is energy within a system. When determining the specifics of internal energy it is best to refer to the real system of the interaction, rather than the point-particle system. The system may consist of internal interactions, such as spring interactions and thermal energy transfer. Internal Energy is any energy in the system other than movement of the center of mass.
Internal Energy is fundamentally Potential Energy, Kinetic Energy, and Rest Energy. Broken down, this includes but is not limited to:
Vibrational Energy: The potential energy of macroscopic springs cannot be modeled as a point particle system, and is often used to represent bonds between atoms in different materials.
Thermal Energy: Thermal Energy is any
energy in the form of heat, or changes in energy from heat transfer.
Rotational Energy: Rotational energy is energy of real system about the center of mass.
Chemical Energy: When a person runs, the change in kinetic energy of the runner is often affiliated with a loss in chemical energy.
A Mathematical Model
Determining change in Internal Energy from Change in Kinetic Energy.
Einternal= Ethermal+Erotational+Evibrational+Echemical+...
Because Energy is conserved if change in total Energy is 0, then:
KEi + Einternal= KEf + Einternal
Thus,
Change in Einternal = -Change inKE
A Computational Model
You can use a computational model to track Kinetic Energy of the point-particle system. Using the change in this Kinetic Energy and the Change in Energy due to the surroundings we can determine the change in Internal Energy of a system.
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Simple
You're pulling a block of mass 20kg across a low-friction floor. While pulling the block you notice the speed of the block has changed from 8m/s to 15 m/s. What was the change in your internal energy over this period of time?
1) Diagram.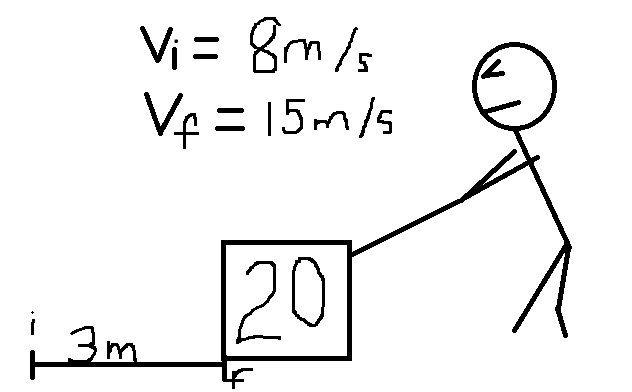 2)Pick your system. (The block). The change in overall energy of the block is equal to the change in kinetic energy: KEf-KEi
2)Pick your system. (The block). The change in overall energy of the block is equal to the change in kinetic energy: KEf-KEi
Change in KE= 1/2*20kg*(15^2-8^2)=1610 Joules
3)Now consider your system to be you, the earth, and the block. Are there any outside forces acting on the system? No. Thus, Change in total energy must be 0.
4)Total Energy= KE+ PE(grav) + Einternal. Since PE(grav) is 0 throughout the interaction and change in total energy is 0, negative change in KE must be equal to your change in internal energy.
Change in your Internal Energy (assuming high efficiency and no energy lost to surroundings) = 1610 Joules
Middling
Difficult
(THIS IS NOT AT ALL PHYSICALLY ACCURATE) Assume you are stranded on an island. You have built a raft with the last of your energy. You have 2 coconuts that will each give you an estimated 1,500 calories of energy. (A calorie is roughly 4.2*10^3 Joules). You have started moving your 200kg raft across the low-friction sand with inital velocity 2m/s. You have calculated that in order to escape the incoming tide, your raft must be travelling at least 12m/s when it reaches the ocean. You hope to save one coconut for your voyage. Will one coconut give you enough energy to move the craft?
Note:Calories is representative of Chemical Energy, and thus find the minimum change in internal energy required to move the raft to solve this problem.
Connectedness
- How is this topic connected to something that you are interested in?
Internal Energy is connected to your everyday life. It fundamentally explains why one must eat (chemical energy) to move (kinetic energy). Internal Energy also describes energy dissipation, and thus is key to solving the Energy crisis.
- How is it connected to your major?
Internal Energy is extremely important to Mechanical Engineering. It explains the different manners and ways that energy can be stored within a system, and later utilized for Kinetic Energy.
- Is there an interesting industrial application?
Using our understanding of Internal Energy, we can minimize Energy dissipation, create better longer lasting batteries, create machines that require less power that can store more energy. Applying our understanding of Internal Energy is our best chance at creating something close to perpetual motion, and maximizing energy output of our systems.
History
Many scientists have contributed to our understanding of Internal Energy. From James Joule's work on thermal energy, to Albert Einstein's famous equation for rest energy.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
This section contains the the references you used while writing this page