Covalent Bonds
Claimed by Elizabeth Apalkova, 7/22/2022
The Main Idea
Covalent bonds are a type of interatomic bond through which two atoms share a pair of valence electrons and become bound to each other as a result. Covalent bonds are intramolecular forces. They are the bonds which hold atoms together while intermolecular forces are the interactions between the molecules. These are the bonds which allow for the formation of the majority of matter on our planet, and carbon's wide variety of possible covalent bond configurations is one of the primary reasons why life was able to eventually spring from it. Covalent bonds can even produce a dipole moment within molecules if an electron is attracted more greatly to one of the two atoms. Molecules made from nonpolar covalent bonds are unable to mix with those made from polar covalent bonds, which is what causes some substances to be hydrophobic, as they are unable to mix with the polarized water molecules.
There are two different types of covalent bonds:
- Polar covalent: electrons are not being equally shared between the atoms. There is a partially positive charge and a partially negative charge that results in the molecule. This phenomena is a result of the difference in electronegativity of the atoms in the bond. The higher the electronegativity of the atom, the more it pulls electrons towards itself and results in a partial negative charge.
- Nonpolar covalent: electrons are being equally shared between the atoms
Both of these bonds can have sigma and pi bond interactions depending on the bond:
- Sigma Bond (σ Bond): Formed by the direct overlapping of electron orbitals on two atoms. This is the strongest type of covalent bond.
- Pi Bond (π Bond): Formed by the lateral overlapping of p or d orbitals on two atoms.
Both polar and nonpolar covalent bonds can have four types of additional covalent bonds:
- Single Bond: The sharing of two electrons between two atoms
- Double Bond: A pairing of one sigma bond and one pi bond, both of which connect the same two atoms together. This bond has electrons being shared
- Triple Bond: A grouping of one sigma and two pi bonds, all of which connect the same two atoms together. This bond has six electrons being shared.
- Half Bond: A bond formed from either one electron being shared between two atoms, or from when an electron shared between two atoms actually disrupts a regular covalent bond, slightly repelling the two electrons in the bond away from each other. These bonds hold half the amount of energy of a single covalent bond between the same atoms.
Strength of bonds
- The bonds that are typically seen are covalent triple, double and single bonds.
- The triple bond is the strongest and shortest bond while the double bond is weaker and longer and the single bond is the weakest and longest bond.
A Mathematical Model
The total energy present within the covalent bonds of a molecule can be found through simply adding together each bond's unique bond energy. For example, water (H2O) contains two H-O bonds, each with a bond energy of 464 kJ/mol, so its total bond energy would be 464 + 464 = 928 kJ/mol. Therefore, it would take 928 kilojoules of energy to break apart the interatomic bonds in a mole of water molecules.
Bonds are also used to calculate the change in enthalpy in a reaction. The change in enthalpy is the bonds broken minus the bonds formed. That can be thought of as the bonds on the reactants side minus the bonds on the products side.
A Computational Model
https://phet.colorado.edu/sims/html/molecule-shapes/latest/molecule-shapes_en.html
- This simulation allows us to see the bonds in real or modeled molecules and how the geometry and angles change as a result of different atoms and different bonds.
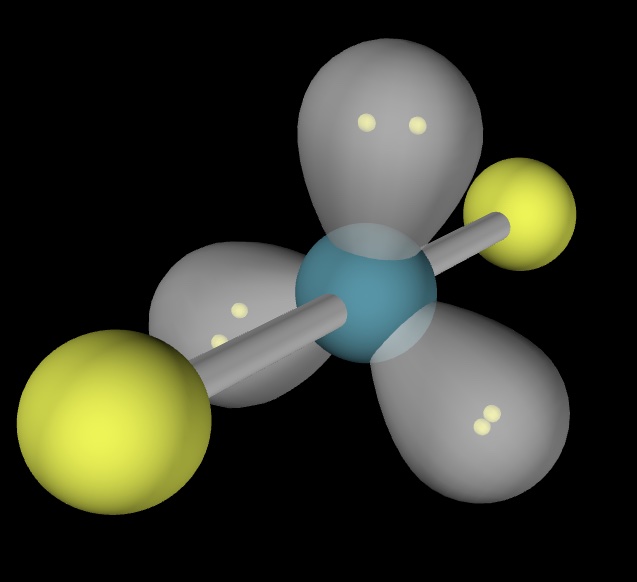
This is an image of the molecule XeF_2
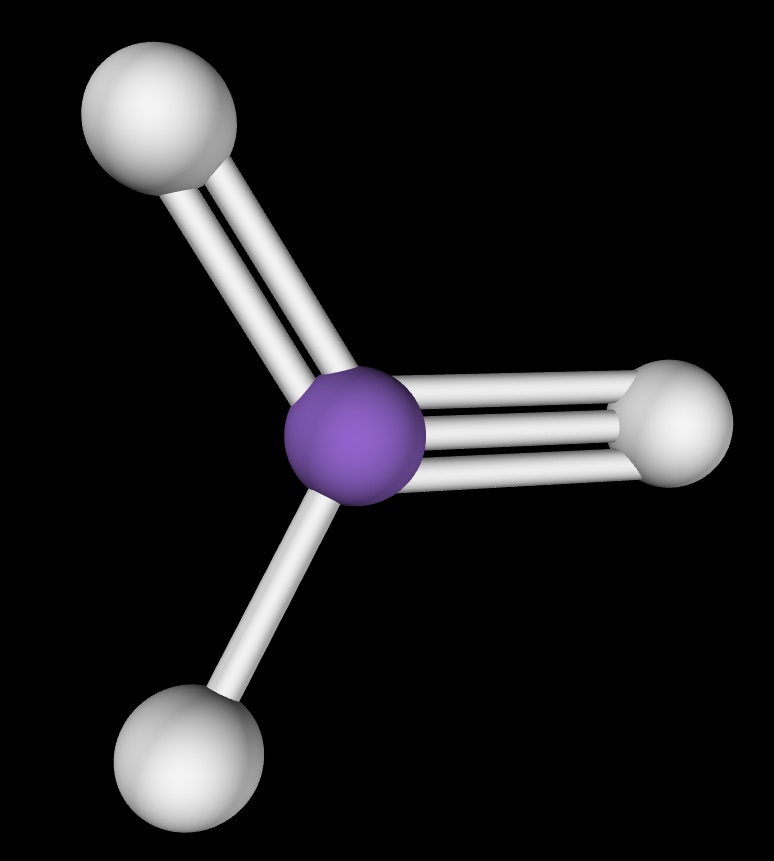
This is an image of a model with one single, one double and one triple bond.
Examples
Simple
Find the total bond energy of an ozone molecule, which has two O=O bonds:
- one O=O bond is 498 kJ/mol
- 498 + 498 = 996 kJ/mol
Middling
Find the total bond energy of a benzene ring, which has 3 C=C bonds, 3 C-C bonds, and 6 H-C bonds:
- one C=C bond is 611 kJ/mol
- one C-C bond is 345 kJ/mol
- one H-C bond is 415 kJ/mol
- 3(611) + 3(345) + 6(415)
- 1983 + 1035 + 2490 = 5508 kJ/mol
Difficult
Calculate the enthalpy change 2C2H6+7O2->4CO2+6H2O In the reactants C-H has 415 kJ/mol
- There are 12 C-H bonds
O=O has 498 kJ/mol
- There are 7 O=O bonds
In the products C=O has 741 kJ/mol
- There are 8 C=O bonds
O-H has 464 kJ/mol
- There are 12 O-H bonds
Enthalpy change = (12(415)+7(498))-(8(741)+12(464)) = -3030 kJ
Connectedness
- How is this topic connected to something that you are interested in?
- I personally have always been fascinated with pharmaceuticals and how so many different and useful drugs can be created through the simple breaking and forming of covalent bonds. For example, the everyday drug that we use as a painkiller, acetaminophen is actually a fairly basic organic compound that can be created by forming and phenol group and an amide with a covalent bond. Also, better understanding of breaking covalent bonds could help us further chemotherapy related drugs to better target only cancerous cells.
- How is it connected to your major?
- The bonding of different atoms together can greatly alter their chemical and physical properties, and thus is responsible for much of the world around us and the events we witness within it. For example, "hydrogen bonding" between water molecules, that being how they attract and repel each other when at different orientations due to their polarity, greatly affects the pattern into which it solidifies, in this specific case causing ice, the solid form of water, to actually be less dense than liquid water. Not only is this an abnormal physical characteristic, it is also likely a major reason complex life was able to develop on Earth, since only the surface of the oceans would freeze during ice ages rather than their entirety, allowing life to grow under the insulating layer.
- Is there an interesting industrial application?
- The ability to trigger the formation of covalent bonds has allowed researchers to synthesize artificial DNA.
- Covalent bonds can also be applied to gases and petroleum. Gas is held together by covalent bonds and our world runs on the use of gases and petroleum.
History
While it was not coined as covalent bonding until 1919 by Irving Langmuir, the theory of the ability for two atoms to share a pair of electrons can be traced back to Gilbert N. Lewis in 1916, who proposed that atoms would bond to each other in order to fill empty energy shells, thus causing them to no longer have any valence electrons. It was also Lewis who created dot notation, commonly used to indicate how many electrons an atom has and how many of those electrons are alone rather than being part of a pair. Much later, in 1927, Walter Heitler and Fritz London proposed the first quantum explanation for covalence, arguing that the bonds are created by the overlapping of electron orbitals.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
These readings delve into intermolecular and intramolecular forces and how these forces have further types with varying strengths based on components such as the types of atoms in the molecule and the electronegativity. https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introduction_to_General_Chemistry_(Malik)/03%3A_Compounds/3.09%3A_Intramolecular_forces_and_intermolecular_forces https://www.masterorganicchemistry.com/2010/10/01/how-intermolecular-forces-affect-boiling-points/#:~:text=There%20are%20four%20major%20classes,of%20%E2%80%9Copposite%20charges%20attract%E2%80%9D.&text=The%20four%20key%20intermolecular%20forces,Van%20der%20Waals%20dispersion%20forces.
External links
https://www.khanacademy.org/science/ap-biology/chemistry-of-life/introduction-to-biological-macromolecules/a/chemical-bonds-article#:~:text=There%20are%20two%20basic%20types%20of%20covalent%20bonds%3A%20polar%20and%20nonpolar. https://chem.libretexts.org/Courses/Furman_University/CHM101%3A_Chemistry_and_Global_Awareness_(Gordon)/04%3A_Valence_Electrons_and_Bonding/4.08%3A_Covalent_Bonding_and_Formula_Writing
References
https://phet.colorado.edu/en/simulations/atomic-interactions/about
http://hyperphysics.phy-astr.gsu.edu/hbase/Chemical/bond.html#c2
https://en.wikibooks.org/wiki/Modern_Physics
https://openstax.org/books/chemistry-atoms-first-2e/pages/9-4-strengths-of-ionic-and-covalent-bonds