Count Alessandro Volta: Difference between revisions
No edit summary |
No edit summary |
||
| Line 27: | Line 27: | ||
In the year 1800, Volta had announced his first invention, known today as the electric battery. At the time, his invention was known as and composed of a concept called the "Voltaic pile". This voltaic pile was the first electrical battery that could provide an electrical current to a circuit. This mechanism consisted of alternating disks of zinc, copper, or silver that was divided by paper, cloth, or cardboard soaked in salt water or sodium hydroxide. The name "Voltaic pile" simply described the physical aspects as it consisted of stacking pairs of alternating disks. With these alternating copper and zinc discs, Volta was able to increase the electrolyte conductivity of the device. When the top and bottom surfaces are connected by wire, an electric current flows through the voltaic pile and the wire itself. This idea formed the basis of all modern wet-cell batteries because it created a new generation of self-sustained electrical current. | In the year 1800, Volta had announced his first invention, known today as the electric battery. At the time, his invention was known as and composed of a concept called the "Voltaic pile". This voltaic pile was the first electrical battery that could provide an electrical current to a circuit. This mechanism consisted of alternating disks of zinc, copper, or silver that was divided by paper, cloth, or cardboard soaked in salt water or sodium hydroxide. The name "Voltaic pile" simply described the physical aspects as it consisted of stacking pairs of alternating disks. With these alternating copper and zinc discs, Volta was able to increase the electrolyte conductivity of the device. When the top and bottom surfaces are connected by wire, an electric current flows through the voltaic pile and the wire itself. This idea formed the basis of all modern wet-cell batteries because it created a new generation of self-sustained electrical current. | ||
[[File:voltaic pile.jpeg| | [[File:voltaic pile.jpeg|450px|center]] | ||
Many new concepts were formed due to Volta's battery: William Nicholson and Anthony Carlisle were able to use the voltaic pile to decompose water into hydrogen and oxygen. With the study of chemistry in their field, they were able to discover the electrolysis of water. Humphry Davy discovered that chemical reaction drove electric current through a circuit containing a single voltaic cell. He was able to demonstrate carbon arc discharge which isolated five new elements currently known on the periodic table as barium, calcium, boron, strontium, and magnesium. William Hyde Wollaston discovered the similarities between electricity generated by the voltaic pile and electricity generated by friction. | Many new concepts were formed due to Volta's battery: William Nicholson and Anthony Carlisle were able to use the voltaic pile to decompose water into hydrogen and oxygen. With the study of chemistry in their field, they were able to discover the electrolysis of water. Humphry Davy discovered that chemical reaction drove electric current through a circuit containing a single voltaic cell. He was able to demonstrate carbon arc discharge which isolated five new elements currently known on the periodic table as barium, calcium, boron, strontium, and magnesium. William Hyde Wollaston discovered the similarities between electricity generated by the voltaic pile and electricity generated by friction. | ||
Revision as of 18:50, 1 December 2015
Claimed by Beiwen Liu
Alessandro Volta was an Italian scientist who invented the first battery that produced a steady current.

Biography
Early Life and Works

Alessandro Giuseppe Antonio Anastasio Volta was a man of many scientific interests. He was considered a physicist, a chemist, and a pioneer of the study of electricity and power. Volta was born in 1745 in Como, Italy and died in 1827. In 1774, he started his career as a physics professor at the Royal School in Como.
By studying electricity on the side, Volta had improved and popularized a device called the Electrophorus, which is a simple capacitive generator that produces electrostatic charge through electrostatic induction. In simple terms, this device generated static electricity. Volta continued to study and experiment with atmospheric electricity, and in 1776, he discovered and found ways to isolate methane gas in the atmosphere. in 1779, Volta was appointed the chair of physics at the University of Pavia.
Mid-Life and Inventions
In 1791, Volta's friend Luigi Galvani, a physicist who also studied electricity, had introduced to him an experiment done with frogs. Volta saw that the contact of two different metals with a particular muscle from the frog created an electrical current. Many interpretations arose as Galvani had named it as "animal electricity", where he believed that electricity could be generated in living tissue, and Volta had named it as "metallic electricity", where he believed that the frog's muscle only served as a conductor when the current flowed between the metals.
Volta came to his conclusions about "metallic electricity" through his experimentation with metals alone as he used metal disks and detected weak flow of electricity simply by placing them on his tongue. As a result, he realized that animal tissue was not a requirement for metal to create a current and that the animal tissue, as well as his tongue, served as conductors.
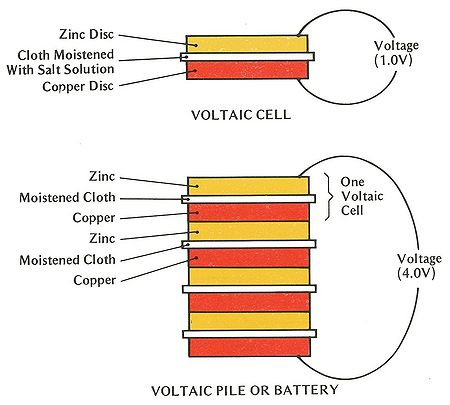
In the year 1800, Volta had announced his first invention, known today as the electric battery. At the time, his invention was known as and composed of a concept called the "Voltaic pile". This voltaic pile was the first electrical battery that could provide an electrical current to a circuit. This mechanism consisted of alternating disks of zinc, copper, or silver that was divided by paper, cloth, or cardboard soaked in salt water or sodium hydroxide. The name "Voltaic pile" simply described the physical aspects as it consisted of stacking pairs of alternating disks. With these alternating copper and zinc discs, Volta was able to increase the electrolyte conductivity of the device. When the top and bottom surfaces are connected by wire, an electric current flows through the voltaic pile and the wire itself. This idea formed the basis of all modern wet-cell batteries because it created a new generation of self-sustained electrical current.
Many new concepts were formed due to Volta's battery: William Nicholson and Anthony Carlisle were able to use the voltaic pile to decompose water into hydrogen and oxygen. With the study of chemistry in their field, they were able to discover the electrolysis of water. Humphry Davy discovered that chemical reaction drove electric current through a circuit containing a single voltaic cell. He was able to demonstrate carbon arc discharge which isolated five new elements currently known on the periodic table as barium, calcium, boron, strontium, and magnesium. William Hyde Wollaston discovered the similarities between electricity generated by the voltaic pile and electricity generated by friction.
The Main Idea
State, in your own words, the main idea for this topic Electric Field of Capacitor
A Mathematical Model
What are the mathematical equations that allow us to model this topic. For example [math]\displaystyle{ {\frac{d\vec{p}}{dt}}_{system} = \vec{F}_{net} }[/math] where p is the momentum of the system and F is the net force from the surroundings.
A Computational Model
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Examples
Be sure to show all steps in your solution and include diagrams whenever possible
Simple
Middling
Difficult
Connectedness
- How is this topic connected to something that you are interested in?
- How is it connected to your major?
- Is there an interesting industrial application?
History
Put this idea in historical context. Give the reader the Who, What, When, Where, and Why.
See also
Are there related topics or categories in this wiki resource for the curious reader to explore? How does this topic fit into that context?
Further reading
Books, Articles or other print media on this topic
External links
Internet resources on this topic
References
This section contains the the references you used while writing this page