Boiling Point: Difference between revisions
No edit summary |
No edit summary |
||
| Line 13: | Line 13: | ||
Boiling point is important in thermodynamics, cooking, meteorology, chemical engineering, distillation, and phase equilibrium. | Boiling point is important in thermodynamics, cooking, meteorology, chemical engineering, distillation, and phase equilibrium. | ||
[[File:Boiling-water. | [[File:Boiling-water.png|center|350px|thumb|Boiling occurs when vapor pressure equals external pressure.]] | ||
--- | --- | ||
| Line 35: | Line 35: | ||
\] | \] | ||
[[File:ClausiusClapeyron.png|center | [[File:ClausiusClapeyron.png|center]] | ||
''Variable definitions:'' | ''Variable definitions:'' | ||
Revision as of 22:02, 1 December 2025
Claimed by Chris Li (Fall 2025)
The Main Idea
The boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure acting on the liquid. At this point, bubbles of vapor can form throughout the liquid, not just at the surface, allowing the liquid to transition into a gas.
Because vapor pressure changes rapidly with temperature, the boiling point is not a fixed property—rather, it depends on:
- **External pressure** (higher pressure → higher boiling point; lower pressure → lower boiling point)
- **Chemical composition** (different liquids boil at different temperatures)
- **Solutes dissolved in the liquid**, which raise the boiling point (a colligative property)
Boiling point is important in thermodynamics, cooking, meteorology, chemical engineering, distillation, and phase equilibrium.
---
A Mathematical Model
Boiling phenomena can be described mathematically using two major relationships:
- **Clausius–Clapeyron Equation** → relates vapor pressure and temperature
- **Boiling Point Elevation Equation** → describes how dissolved solutes raise the boiling point
Clausius–Clapeyron Equation
Used to calculate the boiling temperature at a new pressure when ΔHvap and a reference boiling point are known.
\[ \ln\left(\frac{P}{P_0}\right) = -\frac{\Delta H_{vap}}{R} \left( \frac{1}{T_B} - \frac{1}{T_0} \right) \]
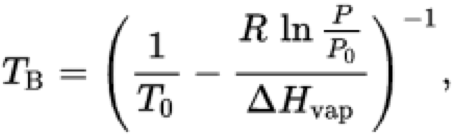
Variable definitions:
- TB – boiling temperature at pressure P
- T0 – known temperature at pressure P0
- P – new vapor pressure
- P0 – reference vapor pressure
- ΔHvap – heat of vaporization
- R – ideal gas constant (8.314 J·mol−1·K−1)
Boiling Point Elevation Equation
Dissolving solute particles raises the boiling point of a solvent:
\[ \Delta T_b = K_b \, b_B \]
Where:
- ΔTb = Tb,solution − Tb,solvent
- Kb = ebullioscopic constant
- bB = effective molality: bsolute · i
- i = van’t Hoff factor
This equation is central in colligative property analysis.
---
A Computational Model
We can simulate boiling point elevation or vapor-pressure curves numerically. The following VPython/GlowScript code models the Clausius–Clapeyron equation to generate a vapor pressure vs temperature plot:
<syntaxhighlight lang="python"> import numpy as np import matplotlib.pyplot as plt
R = 8.314 Hv = 40000 # J/mol T0 = 373.15 # K P0 = 101325 # Pa
T = np.linspace(320, 400, 200) P = P0 * np.exp(-Hv/R * (1/T - 1/T0))
plt.plot(T, P) plt.xlabel("Temperature (K)") plt.ylabel("Vapor Pressure (Pa)") plt.title("Vapor Pressure Curve via Clausius-Clapeyron") plt.show() </syntaxhighlight>
This simulation demonstrates how small temperature changes dramatically affect vapor pressure, explaining why boiling point shifts with altitude and pressure.
A second computational model simulates boiling point elevation:
<syntaxhighlight lang="python"> Kb = 0.512 # water m = np.linspace(0, 5, 200) # molality range i = 2 # NaCl (approx) Tb = 100 + Kb * m * i
plt.plot(m, Tb) plt.xlabel("Molality (m)") plt.ylabel("Boiling Point (°C)") plt.title("Boiling Point Elevation for NaCl in Water") plt.show() </syntaxhighlight>
These visual models help make the equations intuitive.
---
Examples
Below are expanded, step-by-step examples.
Simple
A 1.0 m NaCl solution (i = 2) is prepared in water with Kb = 0.512 °C·kg/mol.
\[ \Delta T_b = (0.512)(1.0)(2) = 1.024^\circ C \]
\[ T_b = 100^\circ C + 1.024^\circ C = 101.024^\circ C \]
Middling
A liquid boils at 360 K under 0.80 atm. What is its boiling temperature under 1.00 atm? Given: ΔHvap = 32 kJ/mol.
\[ \ln\left(\frac{1.00}{0.80}\right) = -\frac{32000}{8.314}\left(\frac{1}{T_B} - \frac{1}{360}\right) \]
Solving gives: \[ T_B \approx 372\ \text{K} \]
Difficult
A liquid has vapor pressure 0.50 atm at 300 K and 1.20 atm at an unknown temperature T2. Use the two-point Clausius–Clapeyron form:
\[ \ln\left(\frac{1.20}{0.50}\right) = -\frac{\Delta H_{vap}}{R}\left(\frac{1}{T_2} - \frac{1}{300}\right) \]
Solving:
\[ T_2 \approx 345\ \text{K} \]
More problems: Boiling Point Elevation
---
Connectedness
Boiling point matters in many real-world contexts:
- **Cooking:** Salt slightly raises water’s boiling temperature; pressure cookers increase pressure to cook food faster.
- **Chemical engineering:** Distillation relies entirely on different boiling points.
- **Meteorology:** Atmospheric pressure affects evaporation and cloud formation.
- **Food production:** Sugar concentration is monitored via boiling temperature in candy-making.
- **Medicine:** Autoclaves use high-pressure steam to sterilize tools.
Boiling point connects physics, chemistry, engineering, and environmental science.
---
History
- **Ancient origins:** Philo and Hero of Alexandria described early thermometric principles and steam devices.
- **1741:** Anders Celsius defined his temperature scale using the boiling and melting points of water (later reversed to the modern form).
- **19th century:** Clapeyron and Clausius formalized the vapor‐pressure–temperature relationship, laying the foundation for phase diagrams and thermodynamics.
The study of boiling was central to the development of thermometers, steam engines, and modern heat science.
---