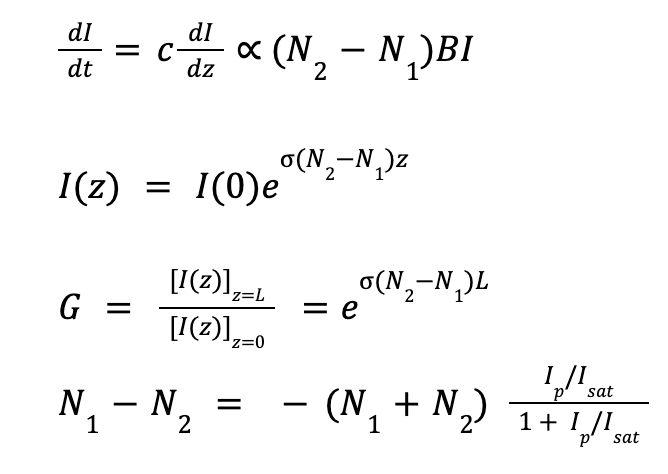Lasers: Difference between revisions
Arappaport23 (talk | contribs) |
Arappaport23 (talk | contribs) |
||
| Line 9: | Line 9: | ||
[[File:Screen_Shot_2022-08-04_at_3.14.34_PM.png]] | |||
Revision as of 14:16, 4 August 2022
Claimed by Adam Rappaport: Summer 2022
Lasers
Lasers have become a very useful invention and have been used in a variety of applications throughout the world, ranging from medical procedures such as laser-eye surgery to simply using a laser to engrave one's initials onto an item of value. The word laser is actually an acronym that stands for light amplification by stimulated emission of radiation, which essentially describes the process by which lasers work.
Lasers excite the atoms in what's called the lasing-medium with either another light or an electric field into a state known as "population inversion" where the majority of the electrons are in an excited state until one of the atoms spontaneously emits a photon. This is caused by an electron dropping from a higher energy state to a lower, more stable energy state. When this photon passes by other atoms of the same material that have electrons that are able to make the same change in energy level (i.e. they could release a photon of the same energy), then they will change energy levels and release a photon, amplifying the light beam by the medium-dependent gain G.[1] This process cascades throughout the medium, emitting more and more photons. Mirrors are typically placed on the sides of the lasing-medium to reflect photons to continue the process, with an opening in one mirror to allow the beam to exit the chamber.[1]
The energy levels mentioned above are relative to each other, and different amounts of energy levels have different uses. Most lasers use atoms that have 3 or 4 energy levels for an electron to jump between.[1] Three-level lasers involve exciting an electron to a higher, unstable state from which of will drop to a slightly lower energy state, called a "metastable state".[1] From this metastable state, the electron can fall to the ground state and release the desired photon. The weakness of three-level lasers is that once the electron is in the ground state, it is then able to absorb a photon, which would prevent the amplification of light by removing photons, so these lasers are usually only good for producing pulses.[1] Four-level lasers work on similar principles as three-level lasers, with a high and unstable state with a slightly lower energy metastable state.[1] The difference is that four-level lasers have an additional state between the metastable state and the ground state called the lower metastable state, which mitigates the issue with the three-level laser.[1] The desired photon is emitted when the electron falls from the higher metastable state to the lower metastable state (rom which the electron may also fall to the ground state).[1] This prevents the electrons in the ground state from absorbing photons since they can only abosorb photons of a discrete energy and there is no energy level above the ground state with that specific energy difference.[1]
Lasers may also be named for the lasing-medium they use, such is the case for "ruby lasers".
Laser Light
To create laser light, there are some conditions that need to be satisfied: the atoms in the lasing-medium must have electrons in an excited state from which they can drop, producing a photon with energy (and thus wavelength) equal to the electron's change in potential energy as it fell, and the light used to stimulate the emission must be that exact same wavelength. This means that all the light emitted by the laser is monochromatic, meaning the photons are all of only one wavelength. [1]
Another difference between laser light and normal light is its state when shone. Laser light is very directional, which explains why when a laser is shone, the beam is very concentrated and organized - there is no or very little scattering of the light. This feat is extremely helpful when considering the applications such as laser-eye surgery where focusing and precise energy is needed.
Here you can find a link to a simulation of a laser that allows you to adjust the parameters of the experiment.
Gain
A device is only classified as a laser if it successfully "lases."
History
The theory of stimulated emission was first suggested by Albert Einstein in 1917. [2] The first person to witness stimulated emission was Rudolf Ladenburg, a german physicist, in 1928. He saw no practical uses for it at the time though, so he effectively dismissed it and lasers went unstudied for over 20 years. [2] This streak came to an end in 1951, when Charles Townes came up with a way to stimulate emission of light in the microwave region of frequencies, and he created a working device in 1953 that he called a "maser" (for microwave amplification through stimulated emission of radiation). [2] Aleksandr M. Prokhorov and Nikolay G. Basov described that theory of the maser independently, ad in 1964, Townes, Prokhorov, and Basov earned the Nobel prize for their work.[2] Townes mentioned the idea of extending the capabilities of the maser to produce stimulated emission of shorter wavelengths of light to his brother-in-law, Arthur Schawlow, and Gordon Gould, who took their research in different directions (Schawlow and Townes began researching optical masers while Gould had more militaristic goals in mind). [2] Gould was the man that came up with the term "laser" but he is not credited with inventing the first one. [2] The first laser was created by Theodore H. Maiman in May of 1960, when he made a laser that used synthetic ruby as the lasing-medium. [2]
Types of Lasers
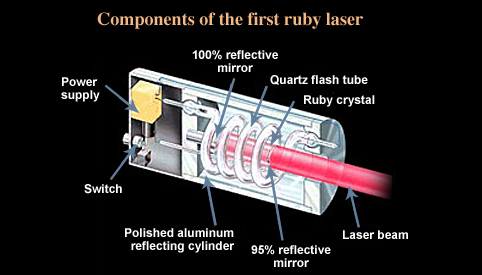
Ruby Lasers
Ruby lasers have a flash tube and two mirrors. In this case, the lasing medium is the ruby rod. When light is flashed, the atoms in the ruby get excited and some of these atoms emit photons, which bounce off the mirrors and are eventually emitted as monochromatic and directional laser.
Solid-state Lasers
In solid-state lasers, the lasing medium is distributed in a solid matrix such as ruby.
Gas Lasers
The Lasing medium in this case is usually either helium or helium-neon. They usually have an output color of red. Carbon dioxide is used as a lasing medium when the lasers are being used to cut hard materials.
Semiconductor Lasers
These are also called diode lasers and are different from solid-state lasers. They are usually very small and use a very small amount of power. These lasers are incorporated into a larger arrays.
Fiber Lasers
Fiber lasers are a variety of solid-state lasers for which an optical fiber doped with a rare earth element, such as cerium, serves as the gain medium. As compared to the majority of lasers, including typical solid-state lasers, fiber lasers generate flatter, smaller, and in turn more precise laser beams. [3]
Liquid Lasers
Connectedness
Lasers are a very unique invention because they have so many applications, as mentioned before. They can be used as a method to decorate objects and have also been applied to the medical field -laser-eye surgery. The concept and process by which lasers work is directly related to physics, specifically the idea of energy emission in the form of photons. This may not have a direct relationship to chemical engineering, but the basic principles of chemistry - atoms and energy - play a large part in the manufacturing of lasers. Industrial applications range from cameras, firearms, scientific techniques such as spectroscopy, and also daily use during presentations. An interesting recent application of lasers is the laser focus that is implemented in LG G3 phone camera. Click on the link below to read more about this feature.
See also
This link has more information about the laser used in the LG G3 phone camera. [ http://www.trustedreviews.com/opinions/how-the-lg-g3-laser-af-camera-focus-works]
References
This section contains the the references you used while writing this page