The Third Law of Thermodynamics: Difference between revisions
Aramirez81 (talk | contribs) No edit summary |
Aramirez81 (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
CLAIMED BY ANTHONY RAMIREZ --- BACK OFF | |||
The Third law of Thermodynamics strives to characterize the low-temperature behavior of a system. O | The Third law of Thermodynamics strives to characterize the low-temperature behavior of a system. O | ||
ne way of interpreting the meaning of the third law is: | ne way of interpreting the meaning of the third law is: | ||
| Line 108: | Line 110: | ||
But because the two curves converge at some non-zero value of entropy,<math>S_o</math>, the amount of entropy expelled from the system becomes less until we are not able to expell any more entropy. | But because the two curves converge at some non-zero value of entropy,<math>S_o</math>, the amount of entropy expelled from the system becomes less until we are not able to expell any more entropy. | ||
This experimental fact | This experimental fact proves Nernst | ||
==References== | ==References== | ||
Revision as of 20:34, 23 April 2022
CLAIMED BY ANTHONY RAMIREZ --- BACK OFF
The Third law of Thermodynamics strives to characterize the low-temperature behavior of a system. O ne way of interpreting the meaning of the third law is:
"It is impossible to reduce the temperature of a system to absolute zero (0°Kelvin) in a finite number of reversible steps"
The Third Law
The Principle of Thomsen and Berthelot
Development of this characterizing principle began in 1906 with Walther Nernst, a chemist from Germany. Nernst developed the "New Heat Theorem" which states: as 0°Kelvin / absolute zero is approached, the change in entropy for a transformation of a system becomes zero. Mathematically, this is,
- [math]\displaystyle{ \lim_{T \to 0} \Delta S = 0 }[/math]
Max Planck proposed a different approach, which would ultimately end up becoming a stronger statement for stating the third law of thermodynamics.His statement can be described as absolute entropy.
Through experimental analysis, Julius Thomsen and Marcellin Berthelot developed a self named principle, stating: chemical changes, which always produce heat, follow a path that leads to maximizing the expulsion of heat / minimizing the chemical/physical systems energy.
In a system, the heat absorbed 'Q' can be described as:
- [math]\displaystyle{ Q = (E_f+P_oV_f)-(E_i+P_oV_i) }[/math]
- [math]\displaystyle{ = H_f - H_i }[/math]
- [math]\displaystyle{ = ΔH }[/math]
This tells us that Enthalpy is equivalent to the heat absorbed 'Q'. Connecting this to the principle of Thomsen and Berthelot tells us that a system will seek a state that minimizes its enthalpy.
When pressure and temperature are held constant, we know for a system to be in equilibrium, the Gibbs free energy must be minimized. Mathematically, this can be described as:
- [math]\displaystyle{ G = E+PV-TS }[/math]
- [math]\displaystyle{ dG = d[E+PV-TS] }[/math]
- [math]\displaystyle{ dG_{PT} \lt 0 }[/math]
This differs from the principle of Thomsen and Berthelot, which says enthalpy must be minimized which is something Nernst was aware of.
Enthalpy, [math]\displaystyle{ H=E+PV }[/math] can be related to the Gibbs free energy, [math]\displaystyle{ G=E+PV-TS }[/math] by recognizing
- [math]\displaystyle{ ΔH = ΔG+TΔS }[/math]
The stability criteria for constant entropy and constant pressure is that the enthalpy be minimized:
- [math]\displaystyle{ H = E+PV }[/math]
- [math]\displaystyle{ dH = d[E+PV] }[/math]
- [math]\displaystyle{ dH_{SP} \lt 0 }[/math]
Therefore, according to [math]\displaystyle{ ΔH = ΔG+TΔS }[/math], when temperature is held constant, enthalpy and gibbs free energy are equal, [math]\displaystyle{ ΔH = ΔG }[/math]. This consideration a lone limits the principle of Thomsen and Berthelot, but when we consider the change in entropy constant as the temperature is held constant, there becomes a way to apply the principle of Thomsen and Berthelot over a variety of temperatures.
Entropy Change
This is the first way Nernst attempted to explain the principle of Thomsen and Berthelot.
He found that as the the change in entropy is zero as the temperature goes towards absolute zero, The entropy change ΔS in any reversible isothermal process approaches zero as the temperature approaches zero.
A change in entropy would mean that as temperature is held constant, certain coefficients used in a characterizing equation of a thermodynamic system will equal zero.
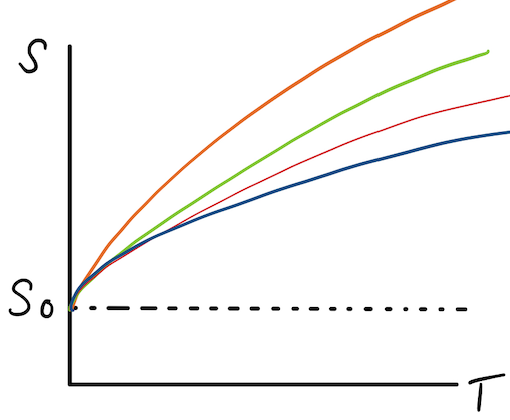
The 'finite entropy hypothesis' states that when entropy is a function of volume and temperature, it will remain finite as the volume is held constant and T approaches zero.
Therefore, according to this hypothesis,
[math]\displaystyle{ S=S(V,T) \to 0 }[/math] as [math]\displaystyle{ T \to 0 }[/math]
Geometrically, what this says is that the slope of graph where entropy as a function of V and T, [math]\displaystyle{ S=S(V,T) }[/math] , when V is constant, will reach a common value of entropy, [math]\displaystyle{ S_o }[/math] when absolute zero is approached.
Unattainability
Nernst would go on to create a statement more refined but equivalent to his original argument of entropy change in order to express the third law as a form of unattainability.
The third law in terms of unattainability states:
No reversible adiabatic process starting at a non-zero temperature can take a system to zero temperature
One example of unattainability can be displayed through adiabatic demagnetization.
Adiabatic Magnetization
Adiabatic magnetization is a process which cools down magnetic objects to very low temperatures. Adiabatic makes reference too the condition that no heat is allowed to escape our system and into the environment or vice versa. To understand this process we must understand Currie's law.
Currie's law of magnetization states the magnetization of a material is equal to a constant times the ratio of an external magnetic field divided by the temperature of the object. Mathematically, we can describe this as:
[math]\displaystyle{ M = σ(\frac{B_{ext}}{T}) }[/math] where [math]\displaystyle{ σ\gt 0 }[/math]
Now before adiabatic magnetization is to be performed, we must first use isothermal magnetization. This means the magnetic field,[math]\displaystyle{ B_{ext} }[/math], is applied while temperature is held constant. Now in order to turn on the magnetic field, we must do work. Doing work relates to a positive quantity. Now because this process is isothermal, the internal energy will change, leading us to make an argument with the first law of thermodynamics:
[math]\displaystyle{ dE=dQ+dW \to dW=-dQ }[/math]
which tells us the system is ejecting heat. When we do adiabatic demagnetization, the temperature of the system winds up having to go down due to energy being a function of temperature. This means that [math]\displaystyle{ Q \to 0 }[/math] due to this process being adiabatic. Therefore in an adibatic magnetization process, [math]\displaystyle{ E=W }[/math].
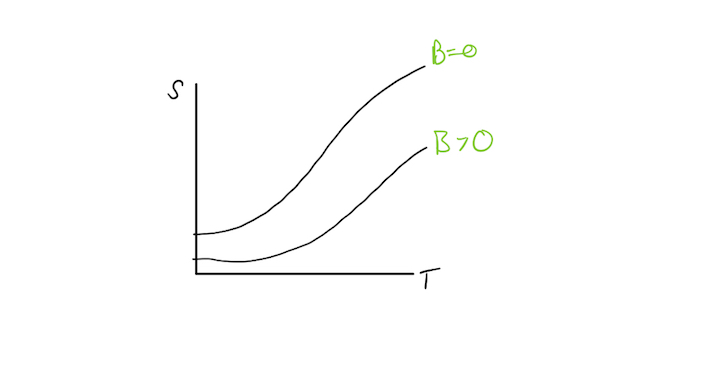
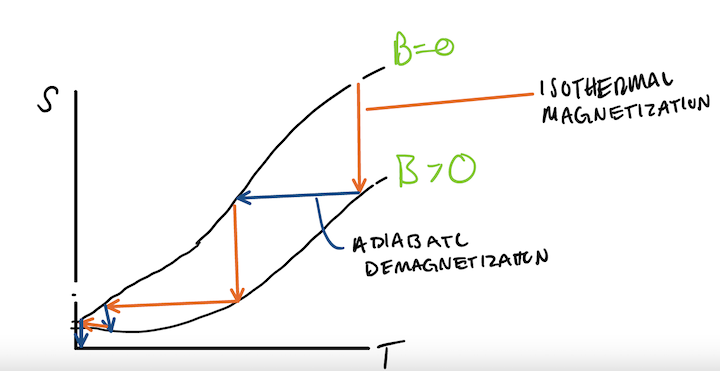
Let us hypothesize an experimental graph of entropy as a function of temperature. Adiabaticc.jpeg
The slope of this graph can be regarded as the magnetic field being applied to the magnet.
So we start at a high temperature, where [math]\displaystyle{ B=0 }[/math] and isothermally magnetize, following the orange arrow on the graph and putting us on the [math]\displaystyle{ B\gt 0 }[/math] curve. This causes the entropy to go down because heat leaves the system which can be proven by the second law of thermodynamics, [math]\displaystyle{ dQ=TdS }[/math].
Next we adiabatically demagnetize the object. Due to this being and adiabatic process, entropy remains constant. This can be displayed by the blue arrows on the graph. This causes us to end back on the [math]\displaystyle{ B=0 }[/math] curve.
Now our temperature has decreased again. We can repeat this process to get to a lower temperature.
But because the two curves converge at some non-zero value of entropy,[math]\displaystyle{ S_o }[/math], the amount of entropy expelled from the system becomes less until we are not able to expell any more entropy.
This experimental fact proves Nernst
References
The New Heat Theorem, Nernst W. https://archive.org/details/in.ernet.dli.2015.206086
Mere Thermodynamics, Don S. Lemons, https://www.goodreads.com/book/show/8359990-mere-thermodynamics