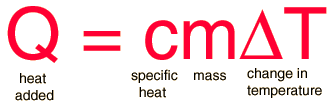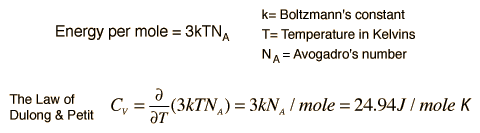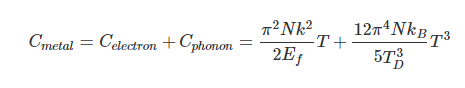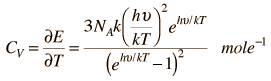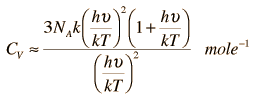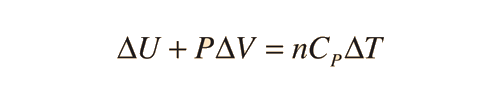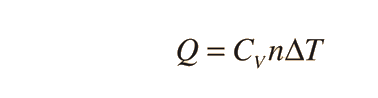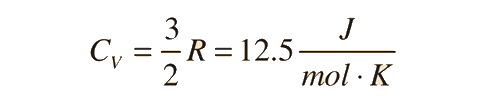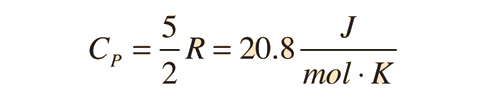Specific Heat: Difference between revisions
Mstevens38 (talk | contribs) No edit summary |
Mstevens38 (talk | contribs) No edit summary |
||
| Line 16: | Line 16: | ||
Q = the amount of heat added to the system (Joules, J) | Q = the amount of heat added to the system (Joules, J) | ||
T = the temperature change (degrees Celsius, ºC) | T = the temperature change (usually degrees Celsius, ºC) | ||
c = the specific heat of the system ([Joules/(gram x degree celsius)], J/gºC) | c = the specific heat of the system ([Joules/(gram x degree celsius)], J/gºC) | ||
| Line 36: | Line 36: | ||
Here is an example of how to calculate specific heat. | Here is an example of how to calculate specific heat. | ||
''' | '''Example: Find the specific heat of 350 g of an unknown substance when 34,700 Joules of heat are applied, and the temperature rises from 22ºC to 173ºC with no phase change.''' | ||
'''We know that''' | '''We know that''' | ||
| Line 64: | Line 64: | ||
[edit] | [edit] | ||
The Law of Dulong and Petit is a thermodynamic law discovered in 1819 by the French physicists Pierre Louis Dulong and Alexis Thérèse Petit. It yields the expression for the molar specific heat capacity of certain chemical elements. They found, through experiments, that the mass specific heat capacity for many elements was close to a constant value, after it had been adjusted to reflect the relative atomic weight of the element. | |||
'''Example''' The specific heat of copper is 0. | Basically, Dulong and Petit found that the heat capacity of a mole of numerous solid elements is about 3R, where R is the universal gas constant. Dulong and Petit were unaware of the relationship to R, since it had not yet been defined. The value of 3R is about 25 joules per kelvin, and Dulong and Petit found that this was the approximate heat capacity of some solid elements per mole of atoms they contained. | ||
'''Example''' The specific heat of copper is 0.389 J/gm K. The specific heat of lead is 0.128 J/gm K. Why are the values so different in these two metals? Did you notice that they are expressed as energy per unit mass? If you express each as energy per mole, they are actually very similar. The Law of Dulong and Petit addresses this similarity in molar specific heats. It can be accounted for by applying equipartition of energy to the atoms of solids. | |||
[[File:Dulong.gif]] | [[File:Dulong.gif]] | ||
| Line 206: | Line 208: | ||
[[Properties of Matter]] | [[Properties of Matter]] | ||
| Line 228: | Line 215: | ||
Random page | Random page | ||
Help | Help | ||
Tools | Tools | ||
| Line 240: | Line 226: | ||
Page information | Page information | ||
This page was last modified on | This page was last modified on 17 April 2016, by Megan Stevens. | ||
This page has been accessed 525 times. | This page has been accessed 525 times. | ||
Privacy policy | Privacy policy | ||
About Physics Book | About Physics Book | ||
Disclaimers | Disclaimers | ||
Revision as of 13:49, 17 April 2016
CLAIMED BY MEGAN STEVENS, being edited right now, 4-17-16
Specific heat, also known as the specific heat capacity, is defined as the amount of heat required to raise the temperature of a unit mass by one degree Celsius. The units for specific heat are Joules per gram-degree Celsius (J / g °C). Specific heat is important as it can determine the thermal interaction a material has with other materials. We can test the validity of models with specific heat since it is experimentally measurable. The study of thermodynamics was sparked by the research done on specific heat. Thermodynamics is the study of the conversion of energy involving heat and temperature change of a system.
The Main Idea
The most common definition is that specific heat is the amount of heat needed to raise the temperature of a mass by 1 degree Celsius. The specific heat of a substance depends on its phase (solid, liquid, or gas) and its molecular structure. The relationship between heat and temperature change is best defined by the constant "c" in the equation below.
where:
m = the mass of the system (grams, g)
Q = the amount of heat added to the system (Joules, J)
T = the temperature change (usually degrees Celsius, ºC)
c = the specific heat of the system ([Joules/(gram x degree celsius)], J/gºC)
The formula below is a reconfigured version of the relationship above. It shows how to calculate "c" specifically from the basic principle.
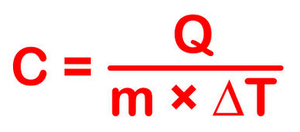
This equation does not apply if a phase change occurs (say from a liquid state to a gaseous state). This is because the amount of heat added or removed during a phase change does not change the overall temperature of the substance. So we disregard this relationship when phase changes take place.
The specific heat for solid can be calculated by the change in energy of the atoms over the change in temperature. The change in energy of the atoms is calculated by dividing the change in the energy of the system by the number of atoms in the substance.
The specific heat most commonly known is the specific heat for water, which is 4.186 J/g°C or 1 calorie/g°C. The specific heat of water is higher than any other common substance. Water has a very large specific heat on a per-gram basis, meaning that it takes a lot more added heat to cause a change in its temperature. Since the specific heat of water is so high, water can be used for temperature regulation. Due to the difference in atomic structures, the specific heat per gram of water is much higher than that of a metal substance. It is possible to predict the specific heat of any material, as long as you know about its atomic structure, as a rise in temperature is the increase in energy at the atomic level of substances. Generally, it is more more useful to compare molar specific heats of substances.
There are two models to determine the specific heats of substances at an atomic level. These are the Dulong-Petit Law and the Einstein-Deybe model. The Dulong-Petit Law states that the molar specific heats of most solids (at room temperature or above) are almost constant. The Einstein-Deybe model of specific heat states that specific heats drop at lower temperatures, as atomic processes become more relevant.
Here is an example of how to calculate specific heat.
Example: Find the specific heat of 350 g of an unknown substance when 34,700 Joules of heat are applied, and the temperature rises from 22ºC to 173ºC with no phase change.
We know that
m = 350 g
Q = 34,700 J
T initial = 22ºC
T final = 173ºC
c = ?
Using the formula above, c = Q / (mΔT)
c = 34,700 / (350*(173-22))
c = 34,700 / (350*151)
c = 34,700 / 52,850
c = 0.657 J/(gºC)
Law of Dulong and Petit
[edit]
The Law of Dulong and Petit is a thermodynamic law discovered in 1819 by the French physicists Pierre Louis Dulong and Alexis Thérèse Petit. It yields the expression for the molar specific heat capacity of certain chemical elements. They found, through experiments, that the mass specific heat capacity for many elements was close to a constant value, after it had been adjusted to reflect the relative atomic weight of the element.
Basically, Dulong and Petit found that the heat capacity of a mole of numerous solid elements is about 3R, where R is the universal gas constant. Dulong and Petit were unaware of the relationship to R, since it had not yet been defined. The value of 3R is about 25 joules per kelvin, and Dulong and Petit found that this was the approximate heat capacity of some solid elements per mole of atoms they contained.
Example The specific heat of copper is 0.389 J/gm K. The specific heat of lead is 0.128 J/gm K. Why are the values so different in these two metals? Did you notice that they are expressed as energy per unit mass? If you express each as energy per mole, they are actually very similar. The Law of Dulong and Petit addresses this similarity in molar specific heats. It can be accounted for by applying equipartition of energy to the atoms of solids.
The specific heats of metals, therefore should all be around 24.94 J/mol degrees Celsius. The specific heat at constant volume should be just the temperature derivative of that energy.
Copper 0.386 J/gm K x 63.6 gm/mol = 24.6 J/mol K
Aluminum 0.900 J/gm K x 26.98 gm/ mol = 24.3 J/mol K
Einstein Debye Model
[edit]
Einstein and Debye had developed models for specific heat separately with Einstien's model saying that low energy excitation of a solid material was caused by oscillation of a single atom, whereas Debye's model stated that phonons or collective modes iterating through the material caused the excitations. However, these two models are able to be extended together to find the specific heat given by the formula:
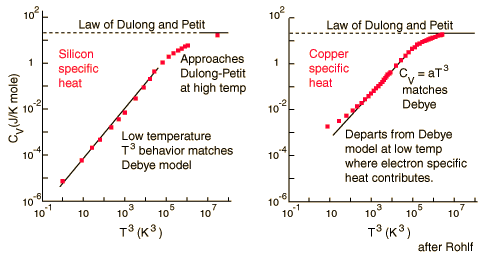
For low temperatures, Einstein and Debye found that the Law of Dulong and Petit was not applicable. At lower temperatures, it was found that atomic interactions were deemed significant in calculating the molar specific heat of an object.
According to the Einstein Debye Model for Copper and Aluminum, two solid metals, specific heat varies much at lower temperatures and goes much below the Dulong-Petit Model. This is due to increased effects on specific heat by interatomic forces. However, for very high temperature values, the Einstein-Debye Model cannot be used. In fact, at high temperatures, Einstein's expression of specific heat, reduces to the Dulong-Petit mathematical expression.
Here is the Einstein Debye Equation:
For high Temperatures it may be reduced like this:
This actually reduces to the Dulong-Petit Formula for Specific Heat:
Specific Heats of Gases
Specific heats of gases are generally expressed in their molar form due to the undefined volume or pressure of a gas. Usually only volume or pressure is held constant at a time. The first law of Thermodynamics helps to derive the formulas for specific heat for constant pressure and the specific heat for constant volume. Here is the equation:
There are two specific heats for gases, one for gases at a constant volume and one gases at a constant pressure. In the formula below, the gas has a constant volume:
where Q is heat, n is number of moles, and delta T is change in Temperature.
For an ideal monatomic gas, the molar specific heat should be around:
For a constant pressure, specific heat can be derived as:
where Q is heat, n is number of moles, and delta T is change in Temperature.
For and ideal monatomic gas, the molar specific heat should be around:
The molar specific heats of gases all gravitate towards these ranges depending on the conditions the gas is kept in.
Connectedness
Specific heat and thermodynamics are used often in chemistry, nuclear engineering, aerodynamics, and mechanical engineering. It is also used in everyday life in the radiator and cooling system of a car.
- Specific heat can have a lot to do with prosthetic manufacturing, which is huge in Biomedical Engineering. Prosthetics materials must be durable and easy to manipulate in a normal range of temperatures. In order to created medical devices, specific heats must be known, especially for welding or molding things, which require a specific temperature to be effective. At higher temperatures, the Dulong-Petit law must be used to calculate the specific heat of an object. Especially for solid metal objects, which would be used in prosthetics, Dulong-Petit is especially useful.
See also
Heat Capacity
External links
References
This section contains the the references you used while writing this page:
http://hyperphysics.phy-astr.gsu.edu/hbase/emcon.html#emcon
http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/spht.html
http://scienceworld.wolfram.com/physics/SpecificHeat.html
http://www.wikihow.com/Calculate-Specific-Heat
http://www.tutorvista.com/content/physics/physics-iii/heat-and-thermodynamics/dulong-and-petit-law.php
Matter & Interactions Vol I. Chabay Sherwood
Main page Recent changes Random page Help
Tools
What links here Related changes Upload file Special pages Printable version Permanent link Page information
This page was last modified on 17 April 2016, by Megan Stevens. This page has been accessed 525 times. Privacy policy About Physics Book Disclaimers