Pauli exclusion principle: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
The Pauli exclusion principle is named after Austrian physicist Wolfgang Pauli. Pauli proposed the assertion in 1925 and received a Nobel Prize in 1945 for his discovery. He is considered a founding father of quantum mechanics and discovered many of his theories and principles without a formal existing definition of what we today call "spin". | The Pauli exclusion principle is named after Austrian physicist Wolfgang Pauli. Pauli proposed the assertion in 1925 and received a Nobel Prize in 1945 for his discovery. He is considered a founding father of quantum mechanics and discovered many of his theories and principles without a formal existing definition of what we today call "spin". | ||
Pauli's discovery and implementation of a fourth quantum number laid the groundwork and inspiration for quantum mechanics in the next years following its announcement. Much of Heisenberg's and Shrodinger's discoveries on wave mechanics were built off of the Pauli exclusion principle. | |||
Revision as of 19:24, 5 December 2015
]Claimed by Michael Segal
The Pauli exclusion principle is a quantum mechanical principle that asserts that no two electrons in the same atom can occupy the same quantum states at the same time.
The Main Idea
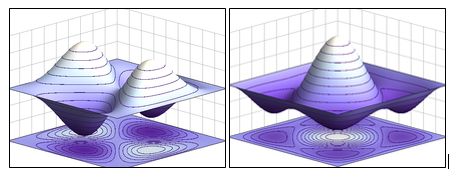
The Pauli exclusion principle asserts that all particles are either fermions or bosons. Fermions have an odd multiple of half spins. Bosons have an even multiple of half spins, thus result in an integer amount of spin. For example, helium-3 is a fermion with the spin of 1/2 and helium-4 is a boson with the spin of 0.
The spins have an intrinsic effect on the angular momentum values of a particle. Particles with a half-integer spin (fermions) have antisymmetric states, while particles with a integer spin (bosons) have a symmetric, wavelike functions.
(Left: Fermion; Right: Boson)
History
The Pauli exclusion principle is named after Austrian physicist Wolfgang Pauli. Pauli proposed the assertion in 1925 and received a Nobel Prize in 1945 for his discovery. He is considered a founding father of quantum mechanics and discovered many of his theories and principles without a formal existing definition of what we today call "spin".
Pauli's discovery and implementation of a fourth quantum number laid the groundwork and inspiration for quantum mechanics in the next years following its announcement. Much of Heisenberg's and Shrodinger's discoveries on wave mechanics were built off of the Pauli exclusion principle.