Robert A. Millikan: Difference between revisions
Miglesias6 (talk | contribs) |
Miglesias6 (talk | contribs) |
||
| Line 12: | Line 12: | ||
[[File:oildrop.jpg|left]] | [[File:oildrop.jpg|left]] | ||
In 1909, Millikan worked with assistant Harvey Fletcher to create the oil drop experiment.The pair were able to find the charge of electron as well as the smallest unit of an electron charged that can be quantized. In this experiment, oil was sprayed onto a plate with a hole. Droplets of oil fell through the whole into a chamber with electrically charged plates. These plates emitted x-rays that caused the negatively charged oil drops to either fall at a slower rate, stop or rise. By comparing the velocity's of the charged and non-charged drops, Millikan and Fletcher found that all the negatively charged particles had a charged that was multiple of 1.6e-19 coloumbs. | |||
===Photoelectric Effect=== | ===Photoelectric Effect=== | ||
Revision as of 02:08, 4 December 2015
By Maria Iglesias

Biography
Robert Andrews Millikan was born March 22, 1868 in Morrison, Illinois. He attended Oberlin College in Oberlin, Ohio, graduating with a degree in classics in 1891. After teaching elementary physics for two years, Millikan went back to school to earn his doctorate in physics from Columbia University. He earned his Ph.D. in 1895, being the first person to do so from that department. Millikan married Greta Ervin Blanchard in 1902. The couple had three children: Clark Blanchard, Glenn Allen, and Max Franklin. In 1908, he became an assistant at the University of Chicago where he later became a professor. Millikan later went on to be the director of the Norman Bridge Laboratory of Physics at the California Institute of Technology in 1921. Robert Millikan died on December 19, 1953 in San Marino, California at the age of 85.
Major Scientific Contributions
Oil Drop Experiment
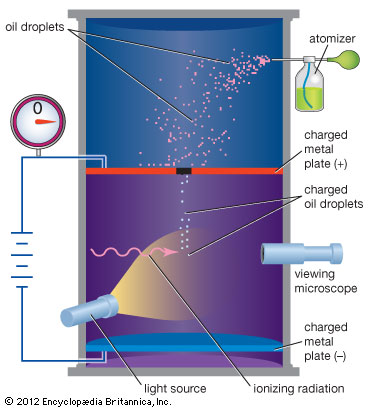
In 1909, Millikan worked with assistant Harvey Fletcher to create the oil drop experiment.The pair were able to find the charge of electron as well as the smallest unit of an electron charged that can be quantized. In this experiment, oil was sprayed onto a plate with a hole. Droplets of oil fell through the whole into a chamber with electrically charged plates. These plates emitted x-rays that caused the negatively charged oil drops to either fall at a slower rate, stop or rise. By comparing the velocity's of the charged and non-charged drops, Millikan and Fletcher found that all the negatively charged particles had a charged that was multiple of 1.6e-19 coloumbs.
Photoelectric Effect
How do we visualize or predict using this topic. Consider embedding some vpython code here Teach hands-on with GlowScript
Fun Facts
Be sure to show all steps in your solution and include diagrams whenever possible
External links
References
This section contains the the references you used while writing this page