Malleability: Difference between revisions
Ehermosilla3 (talk | contribs) |
Ehermosilla3 (talk | contribs) |
||
| Line 37: | Line 37: | ||
[[File:MalleabilityDuctilityTest.gif]] | [[File:MalleabilityDuctilityTest.gif]] | ||
Because there is no definitive method or unit for measuring malleability, oftentimes metals are simply compared to each other to create a scale of malleability, as detailed in the | Because there is no definitive method or unit for measuring malleability, oftentimes metals are simply compared to each other to create a scale of malleability, as detailed in the examples section below. However, since malleability is so closely connected with a metal's hardness, material scientists look to hardness tests, such as the Rockwell Test, to predict a metal's malleability. See the section below on the Rockwell Test for additional information. | ||
===Measuring Hardness with the Rockwell Test=== | ===Measuring Hardness with the Rockwell Test=== | ||
Latest revision as of 04:04, 22 April 2024
This page covers one of the intensive properties of matter: Malleability
by Elizabeth Hermosilla (Spring 2024) Previous authors: Marguerite Murrell Kyle Williams
The Main Idea
A Property of Matter
Properties of matter can be broken down into two distinct categories: physical and chemical. The physical category can also be broken down in a similar manner, consisting of intensive and extensive properties. A physical property is one that can be determined without changing the identity of the substance, as opposed to the process of identifying chemical characteristics which require preforming chemical reactions. Intensive properties can be determined regardless of the amount of matter of the substance present whereas extensive properties, like mass and volume, depend on the amount of the material. Malleability is classified as a property of matter, along with ductility and conductivity.
What is Malleability?
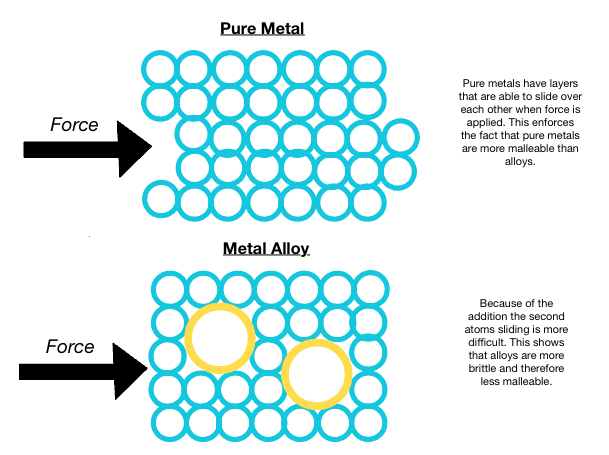
Malleability is the ability for something, generally metals, to be molded or deformed into another shape. Often considered to simply be the ability for a metal to be hammered into thin sheets, malleability is actually a material's ability to deform under pressure of a force pushing on it, in other words, a compressive force. Malleability certainly also has a close relationship with an object's hardness, which is defined as a material's resistance to being indented. Harder objects are considered less malleable. Metal alloys are normally brittle relative to pure metals.
How Does it Work?
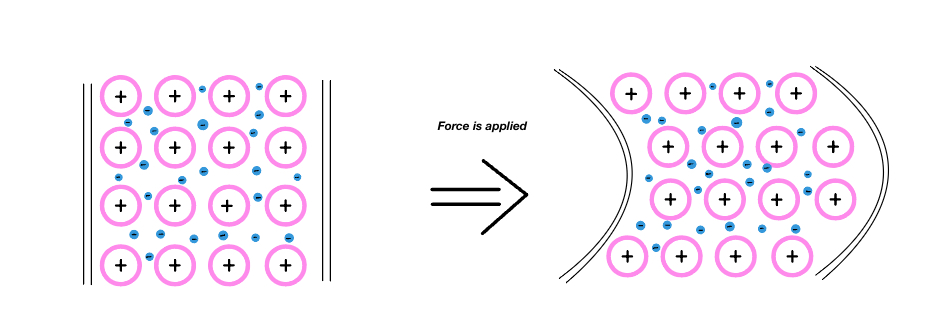
Malleability in metals is due to metallic bonds which are characterized by a mobile "electron sea". The electrons are able to move around and allow the metal atoms to adjust back and forth, past other atoms if a force is applied to them. The atoms in a metal are packed tightly, fitting as many as possible in the metal's respective space/volume, creating layers of atoms side by side, sometimes aligning. Areas where atoms align are called "crystal grains".
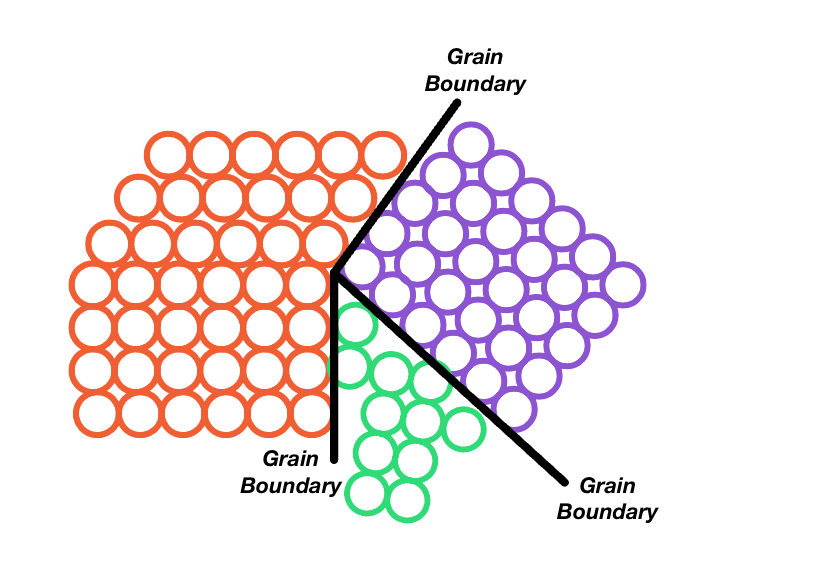
A metal is more likely to break at the grain boundaries (the point where crystal grains meet), so metals with a higher grain boundary count and larger grain boundaries is considered less malleable. The actual deformation of a metal occurs when pressure/stress are put on the object, causing the atoms to roll over one another and permanently settle in a different place.
The amount the atoms move is dependent upon two key factors: the temperature and the strength of the metallic bonds. Having weak metallic bonds means that there is less energy required to move the relative positions of the atoms and therefore means the material will have a higher malleability. Likewise, a metal or alloy with a stronger metallic bond needs more energy to change its atom's alignment. Temperature affects the malleability of a material by regulating the crystalline structure of the atoms. In most metals, the heat makes the structure of the atoms more regular which softens the metal and makes it more malleable.
Malleability and Ductility
It is important to keep in mind that malleability is not the same as ductility. These two physical properties of metals, although similar, have a few distinct differences. Ductility is the ability of a material to stretch under tensile stress, a force acting away from the object. Malleability, on the other hand, is a metal's ability to deform under compressive stress, a force acting towards the center of the object. The key difference here, is tensile versus compressive stress. Some exceptional materials, such as copper, have both good ductility and good malleability.
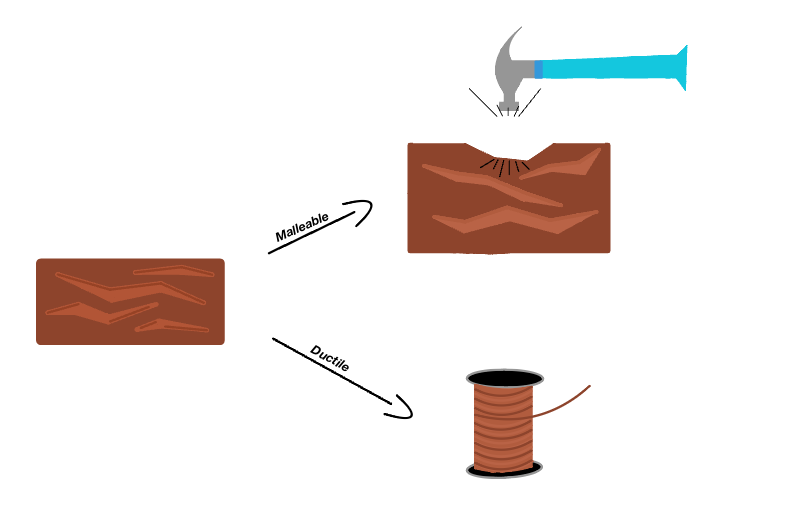
Measuring Malleability
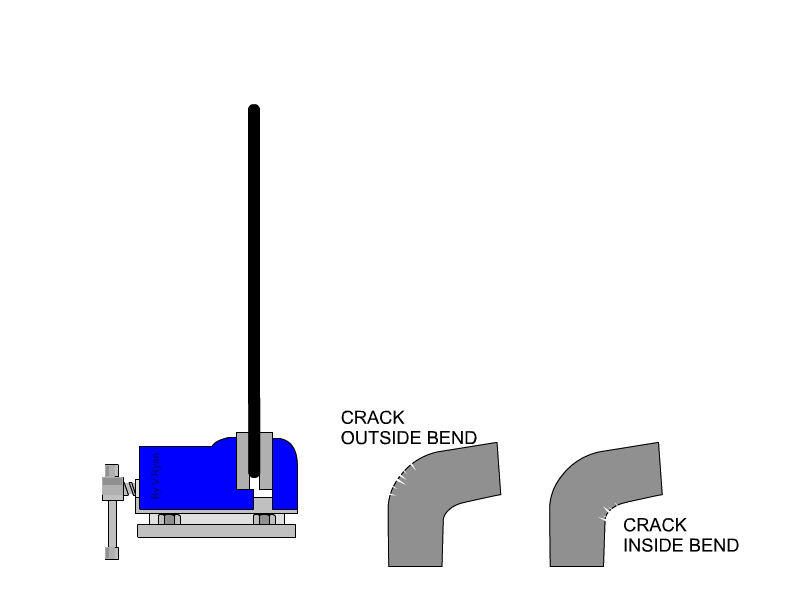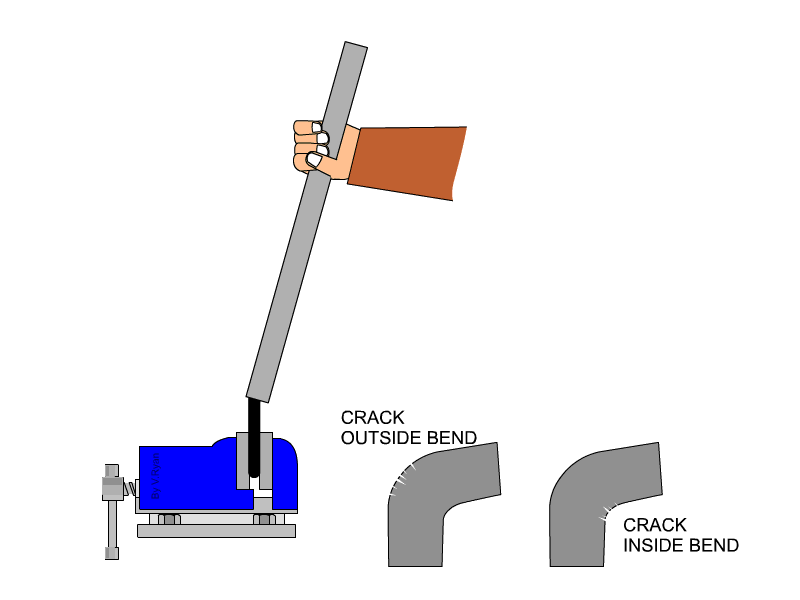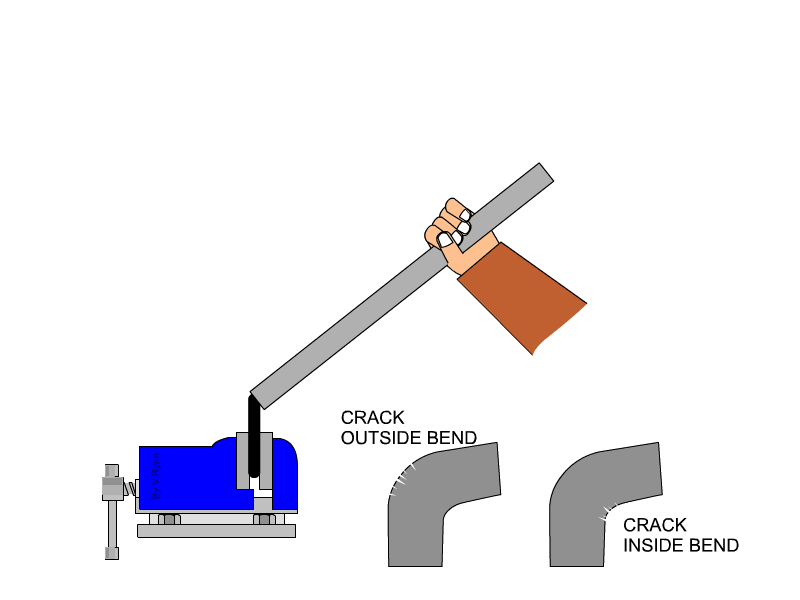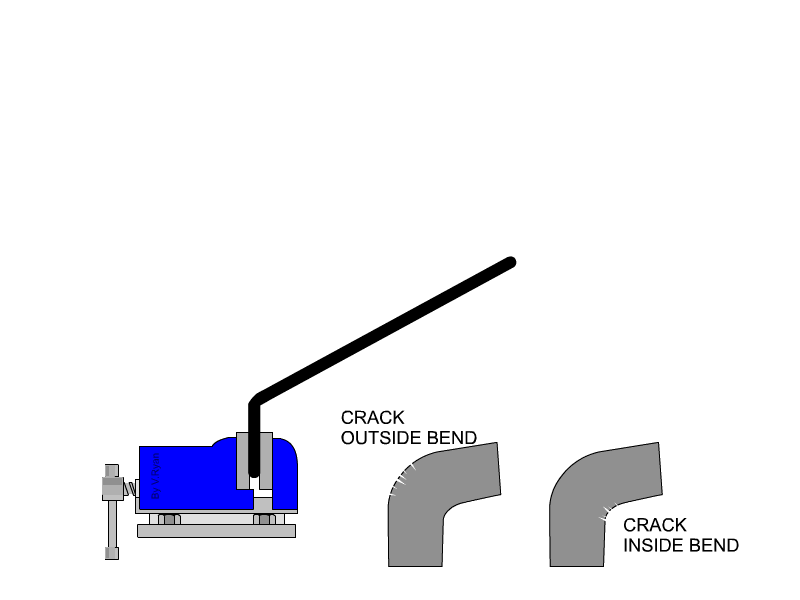
Malleability is not typically or easily measured with quantitative values. We determine a metal's malleability by testing how much stress it can take before breaking, and additionally testing whether a metal can be rolled into a sheet (some metals can be flattened into thinner sheets than others). Both malleability and ductility can be tested at the same time by bending a rod of the material of discussion until it cracks at the place of bend. Cracks on the outside of the rod indicates how ductile the material is, because that area of the material is being stretched as the rod bends. Cracks on the inside of the bend indicate the material's malleability, because that area is being compressed as the rod is bent.
Because there is no definitive method or unit for measuring malleability, oftentimes metals are simply compared to each other to create a scale of malleability, as detailed in the examples section below. However, since malleability is so closely connected with a metal's hardness, material scientists look to hardness tests, such as the Rockwell Test, to predict a metal's malleability. See the section below on the Rockwell Test for additional information.
Measuring Hardness with the Rockwell Test
The Rockwell Test is debateably the most common test to measure how "hard" a material is - that is, how resistant to indentation it is. Since hardness is not a physical property of materials, just a characteristic, it is not formally used to determine a material's malleability or ductility. However, in the material science world, the Rockwell Test is a great indicator to whether or not a material could be malleable, since the act of indentation is a form of compressive stress. The Rockwell Test is conducted as follows:
First, a preload (preliminary test force) is applied to the sample metal using a diamond indenter, because diamonds are the hardest material we know of. This indentation is known as the reference position. Second, the major load, or main load force, is applied to the metal. This force is made sure to be applied for a certain amount of time in case a certain metal is highly elastic and the indentation does not remain permanent (the Rockwell Test is testing for permanent indentation of a metal). Lastly, the hardness level is obtained by calculating the difference in position of the preload and the major load, and then converting the number to a value on the Rockwell Scale.
The Rockwell Scale and final value that describes a material involves both numerical values and letters on a scale from A - G. The final hardness level generated by this test comes from a combination of information including the size of the force load, the material of the indenter (most commonly diamond or steel), and the depth of indentation. There are several other reliable hardness test methods, but the Rockwell Test is the most commonly used and praised for its reliability, speed, and preservation of the test material since the indentation area is typically very small.
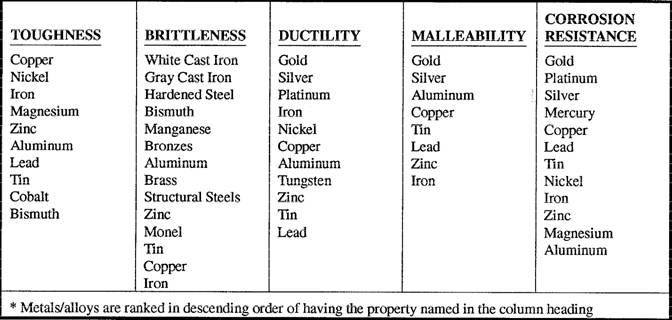
Examples
Scale of Malleability
Since there is no universal quantitative way of measuring a material's malleability, it is determined based on comparison to other like materials. View the list below for a scale of malleability (from most malleable to least) among common alloys and metals:
Products and Common Use
While malleability can be considered to some extent with everything you see everyday, one of the most common everyday uses of malleability is with aluminum foil. Whether for a science project or for leftovers from dinner, the ability to crumple up or change the shape of aluminum foil is quite convenient. Pottery, horseshoes, and swords/weapons are also common examples of malleability put to work. Materials with high malleability, such as gold, silver, and chrome, can be used for dental fillings, creating electronic components for computers and cell phones, jewelry, and decoration.

-
Architectural Use
Since gold is the most malleable material in existence and can be flattened into sheets as thin as a few atoms thick, gold leaf is commonly used for decoration of important monuments, statues, government buildings, and royal homes. Examples in history include much of the gold decor in King Louis XIV's Palace of Versailles, where gold was used as a motif for the Sun King, and integrated in almost every room of the palace, as well as the exterior and gates. Additionally, centuries later, gold leaf is still popular in architecture, for example on the dome on the Georgia State Capitol building. The decorative process of coating with gold leaf is an art called gilding, during which one can really see just how malleable the decorative material is: [1]


-
In addition to the use of golf leaf, other malleable materials are immensely used in architecture, like iron for wrought iron gates, and steel for foundation beams in buildings. Typically, stronger materials that are flexibility (which is depending on ductility), are used for the structural foundation of buildings, like steel and iron. Silver, gold, and chrome are extremely malleable and are thus used for decorative purposes more often.
Industrial Applications
The property of malleability is utilized in industrial applications through processes such as forging, drop-stamping, and hot-rolling. These and many other processes allow different metals to be worked and formed into all sorts of useful items. Steel-girder bridges, one of the most common types of modern bridges used, are an interesting application of this property. The steel grid helps support the concrete deck that serves as the walkway or roadway surface. The supporting structure of the bridge consists mainly of the steel holding up the deck. Without malleability, the beams used for these types of bridges wouldn't be able to be forged and stamped, nor would they be able to withstand the compression that the load on the bridge itself causes.

History
Derived from the Medieval Latin word, malleābilis (almost directly meaning "hammer-able"), malleability has been understood and utilized for centuries for a variety of things. Such uses range from the molding of clay for pottery to the forging of swords and armor in Medieval times, and even to the production of steel beams used in the construction of modern bridges. The modern era of chemistry and physics, however, has allowed a more controlled use of this intensive property of matter in industrial applications.
See also
Ductility and Conductivity
External links and Further Reading
http://metals.about.com/od/metallurgy/a/Malleability.htm
http://www.boeingconsult.com/tafe/structures/struct1/Stress-strain/stress-strain.HTM
References
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Properties_of_Matter
http://study.com/academy/lesson/malleability-in-chemistry-definition-examples-quiz.html
https://en.wikipedia.org/wiki/Girder_bridge
http://www.chemguide.co.uk/atoms/structures/metals.html
https://www.thebalance.com/malleability-2340002
http://www.tpub.com/steelworker1/2.htm
http://www.technologystudent.com/joints/conduct1.html
http://www.hardnesstesters.com/Applications/Rockwell-Hardness-Testing.aspx